Research in the CCHF:
The C–H functionalization approach embodies many of the drivers that motivate modern science and can be summarized as a truly sustainable strategy by four key statements;
1. As a primarily catalytic transformation only very small amounts of high value reagents (catalysts) are required to convert large amounts of feedstock chemicals into essential commodities.
2. Employing a C–H bond as the reaction partner removes the need for introduction or inter-conversion of functional groups, significantly expanding the scope of feedstock chemicals available for reaction and thus
3. considerably reducing the number of operations required to achieve a desired molecular change.
4. A meaningful reduction in the volume of hazardous waste generated by using the C–H bond as a reaction partner (typical byproducts include hydrogen and nitrogen gas or water).
Three strategies have been identified by the Center to impart selectivity in C–H Functionalization processes:
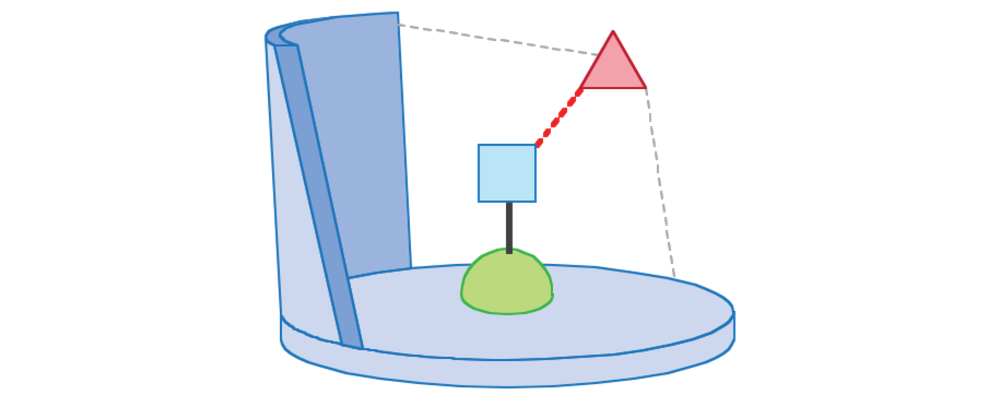
- Catalyst Controlled Site Selectivity: The design and synthesis of organometallic complexes with ligand architectures effective at guiding the substrate to the active site through steric and electronic interactions.
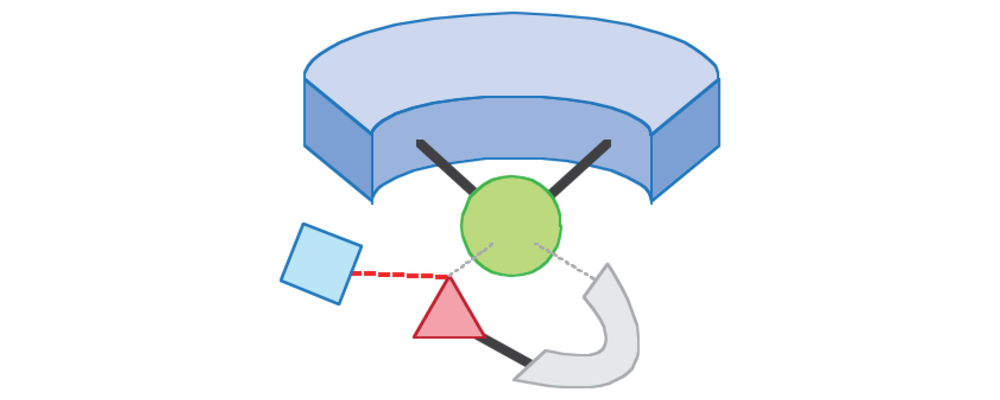
- Substrate-Chelation-Assisted Site Selectivity: Design and coordination of a transient assembly of substrate, organometallic complex and ‘template ligand’ that come together in a specific geometry to deliver the reactive site to a specific C–H bond. This strategy takes advantage of weak electronic coordination between a function group on the substrate and the catalyst / ligand assembly.
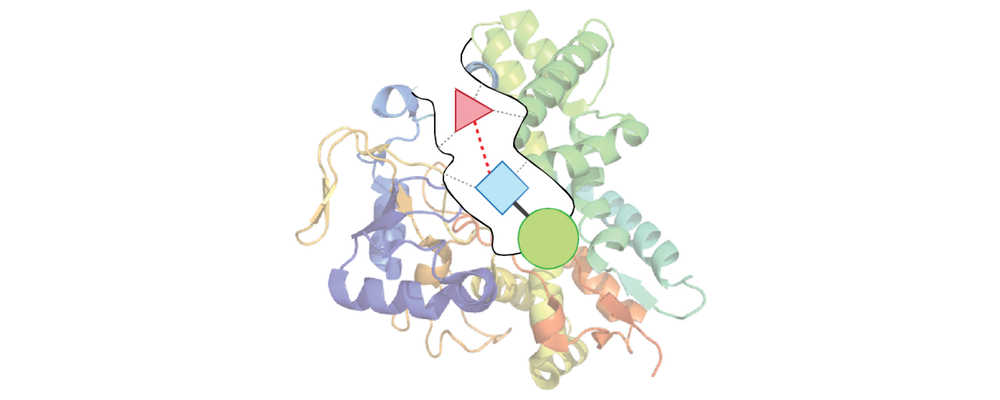
- Bio-Inspired Catalyst Site Selectivity: Using a combination of protein and substrate engineering to exploit the reactivity and exquisite selectivity of Nature’s enzymes to perform novel C–H functionalization reactions on a broad substrate scope.
The image below summarizes how the different research themes engage and interact with each other in our collaborative community.
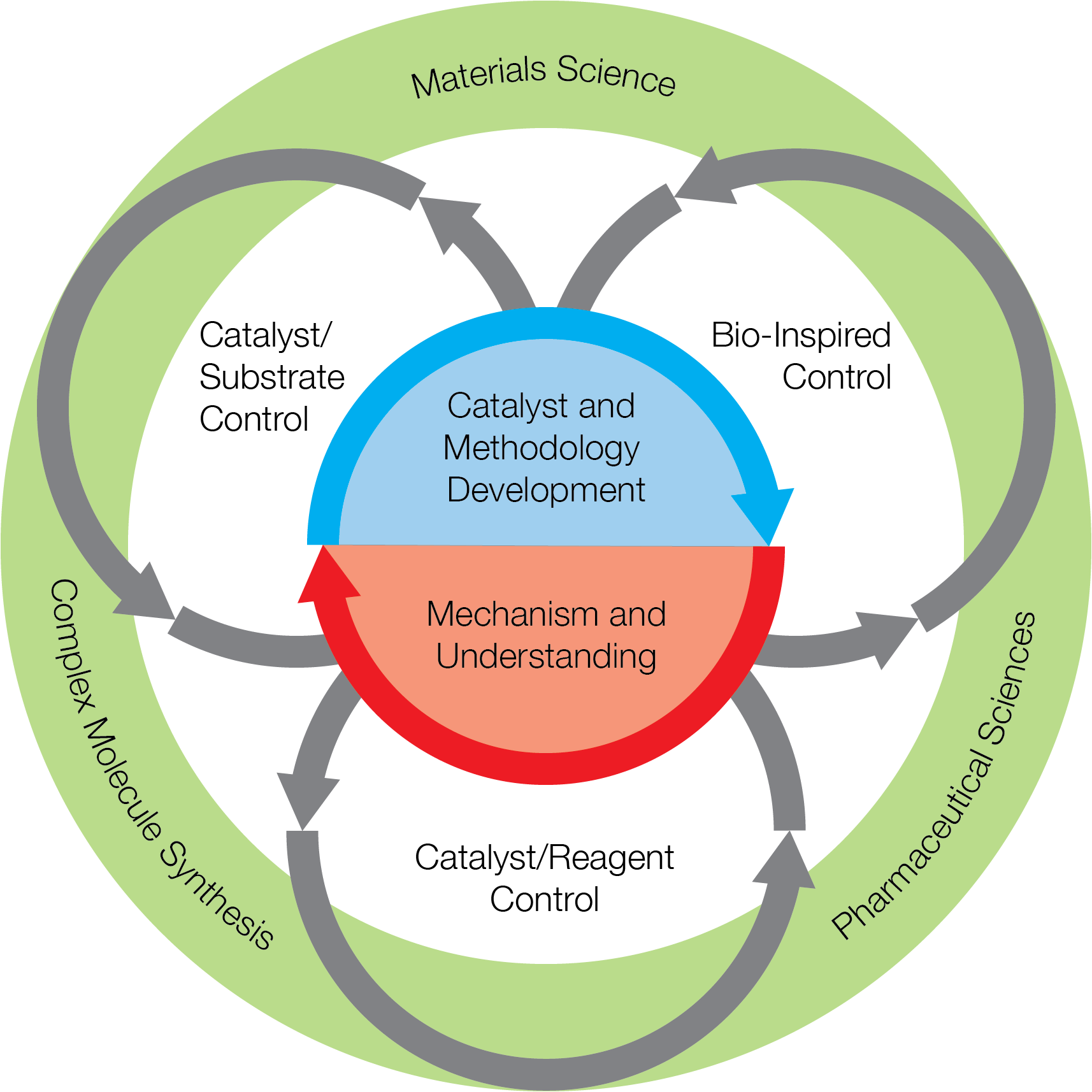
CCHF Selectivity Strategies:
Themes within Center Research:
-
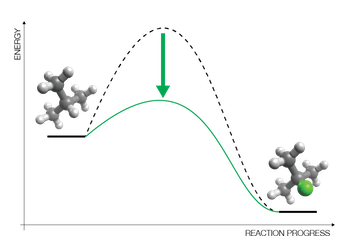
Catalyst Design
RESEARCH
-
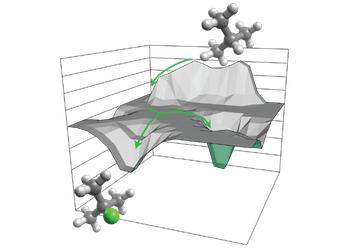
Mechanistic and Theoretical Studies
RESEARCH
-
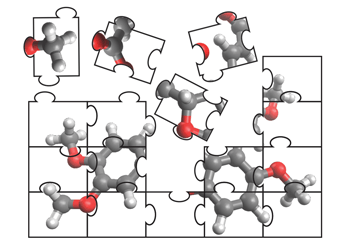
Novel Disconnection Strategies
RESEARCH
-
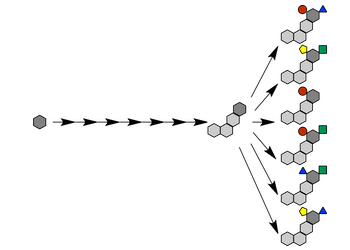
Late-Stage C–H Functionalization
RESEARCH
-
Applications in the Material Sciences
RESEARCH
-
Applications in the Pharmaceutical Sciences
RESEARCH