CCHF Research | Catalyst Design
Led by Justin Du Bois
The ability to exact control over product selectivity in molecules possessing multiple, disparate C–H bonds is arguably the defining challenge for C–H functionalization research. Over the past several years, CCHF researchers have had a leading role in establishing intrinsic patterns of C–H bond reactivity and in demonstrating the influence of substrate structure and functional group substitution on reaction outcomes.
Differential rates of C–H functionalization are evident in all molecules, but such dissimilarities are typically small and often insufficient to bias reactions towards a single product outcome. Accordingly, the ultimate objective in C–H functionalization research is to control product selectivity (i.e., chemo-, regio-, stereo-) through judicious reagent and catalyst selection. Within the collaborative environment of CCHF, the need for such solutions has become even more acute, as substrates designed by Center members en route to natural products and active pharmaceutical ingredients are ever-more complex.
A primary goal of CCHF is to produce a ‘guidebook’ for identifying catalyst, reagent(s), and reaction conditions that would enable specific control over site- and stereoselectivity in all types of C–H functionalization reactions, thus empowering chemists who wish to employ such technologies for streamlining chemical synthesis and/or for the rapid structural diversification of chemical entities. To realize this ambitious challenge, the Center is leveraging the talents of multiple research labs that span analytical, mechanistic, biochemical, inorganic, synthetic, materials, and theoretical chemistry along with chemical engineering.
Related Publications:
-
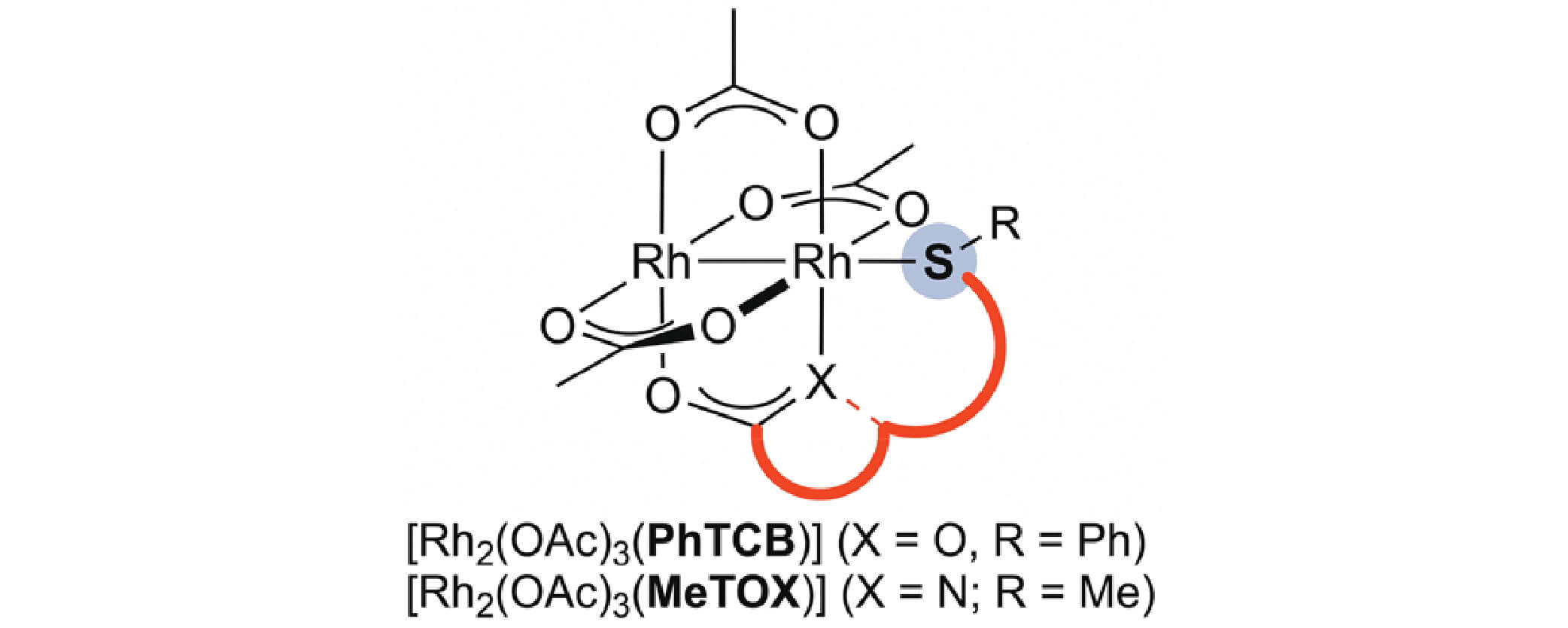
01/2019
Synthesis and Catalytic Properties of Dirhodium Paddlewheel Complexes with Tethered, Axially Coordinating Thioether Ligands
RESEARCH
-
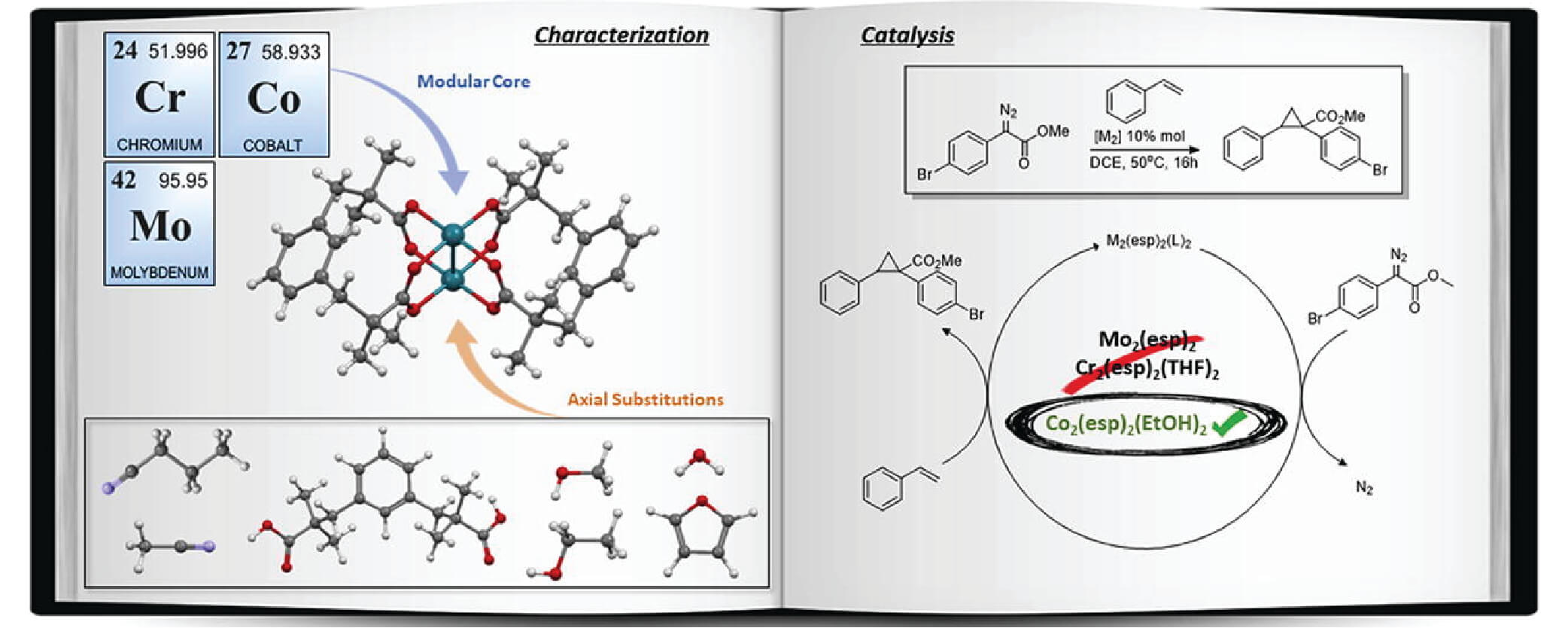
01/2019
New chromium, molybdenum, and cobalt complexes of the chelating esp ligand
RESEARCH
-
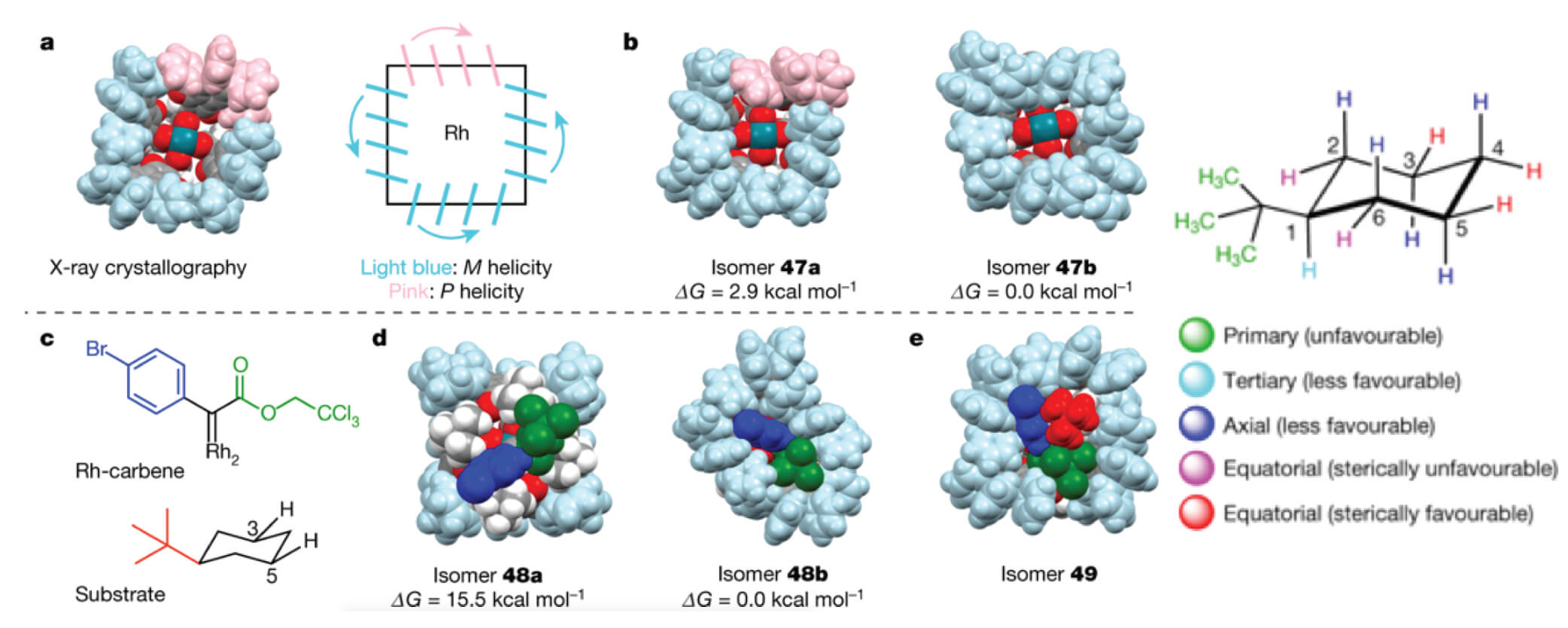
12/2018
Desymmetrization of cyclohexanes by site- and stereoselective C–H functionalization
RESEARCH
-
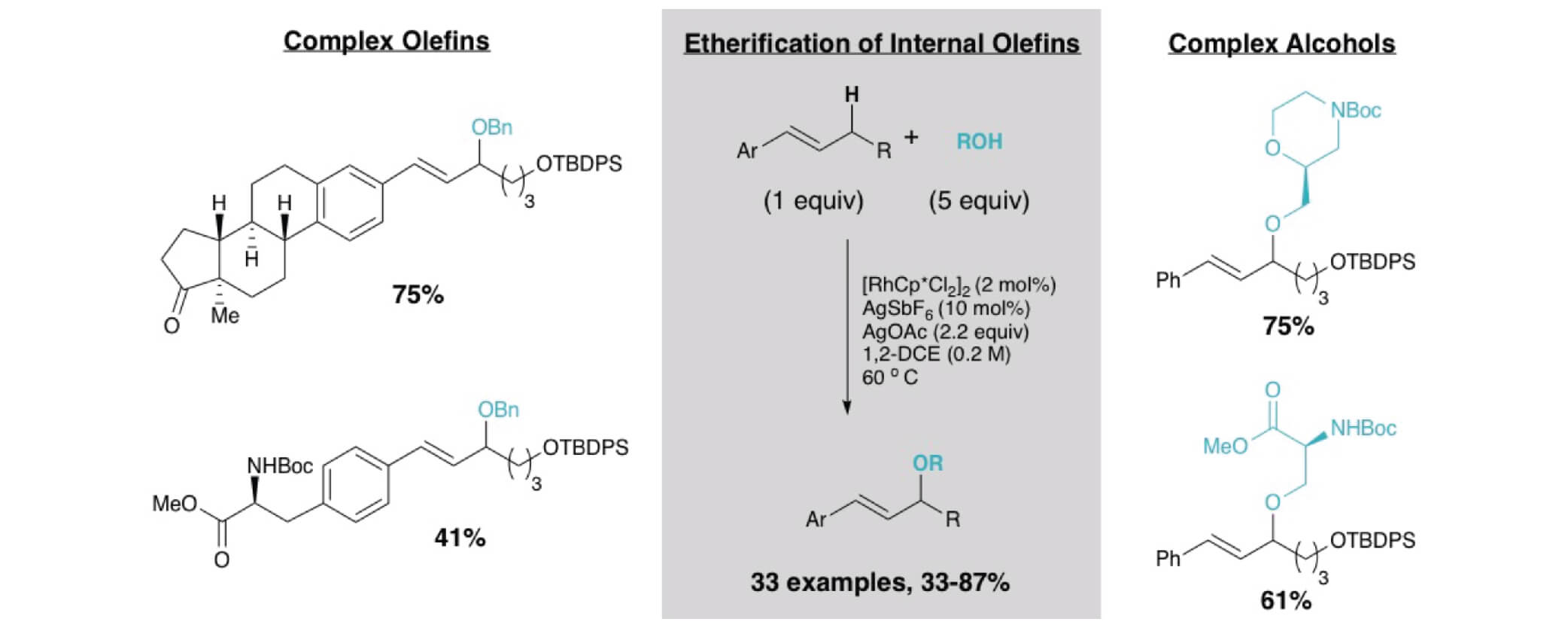
09/2018
Intermolecular Allylic C−H Etherification of Internal Olefins
RESEARCH
-
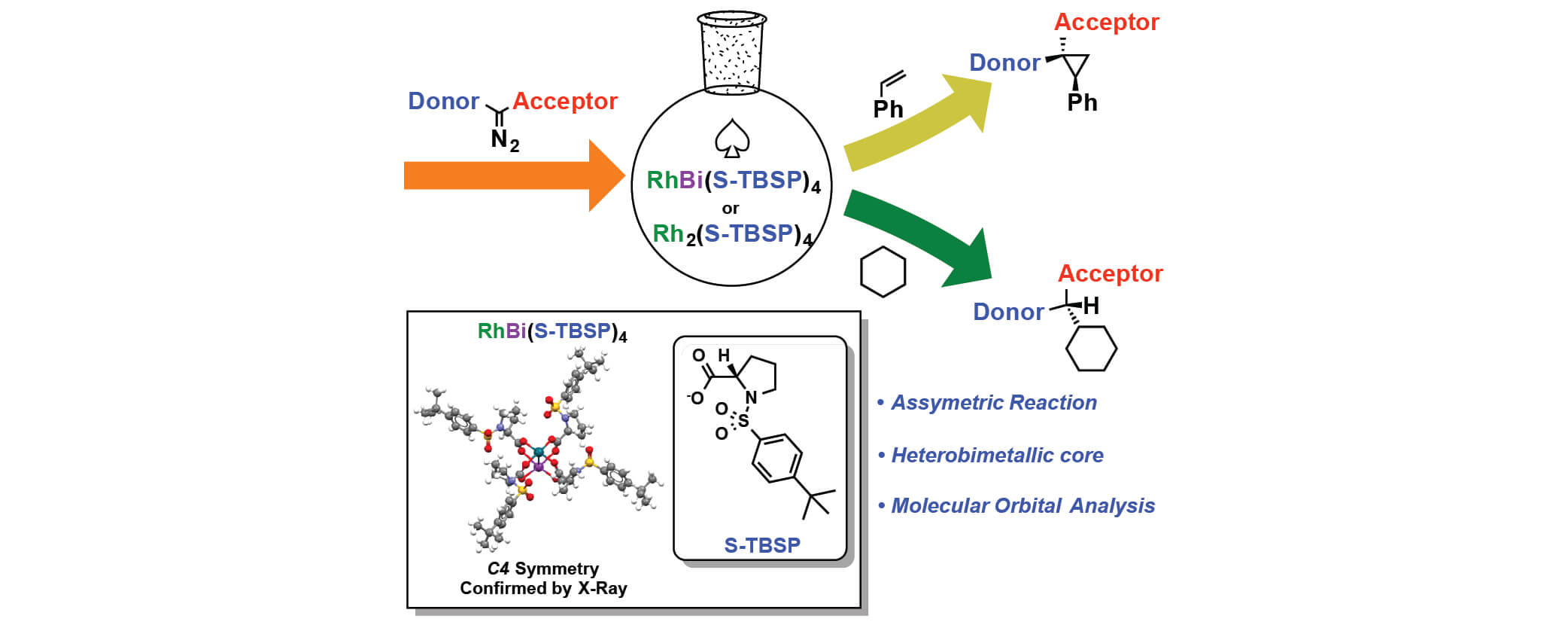
09/2018
Comparison of Reactivity and Enantioselectivity between Chiral Bimetallic Catalysts: Bismuth–Rhodium- and Dirhodium-Catalyzed Carbene Chemistry
RESEARCH
-
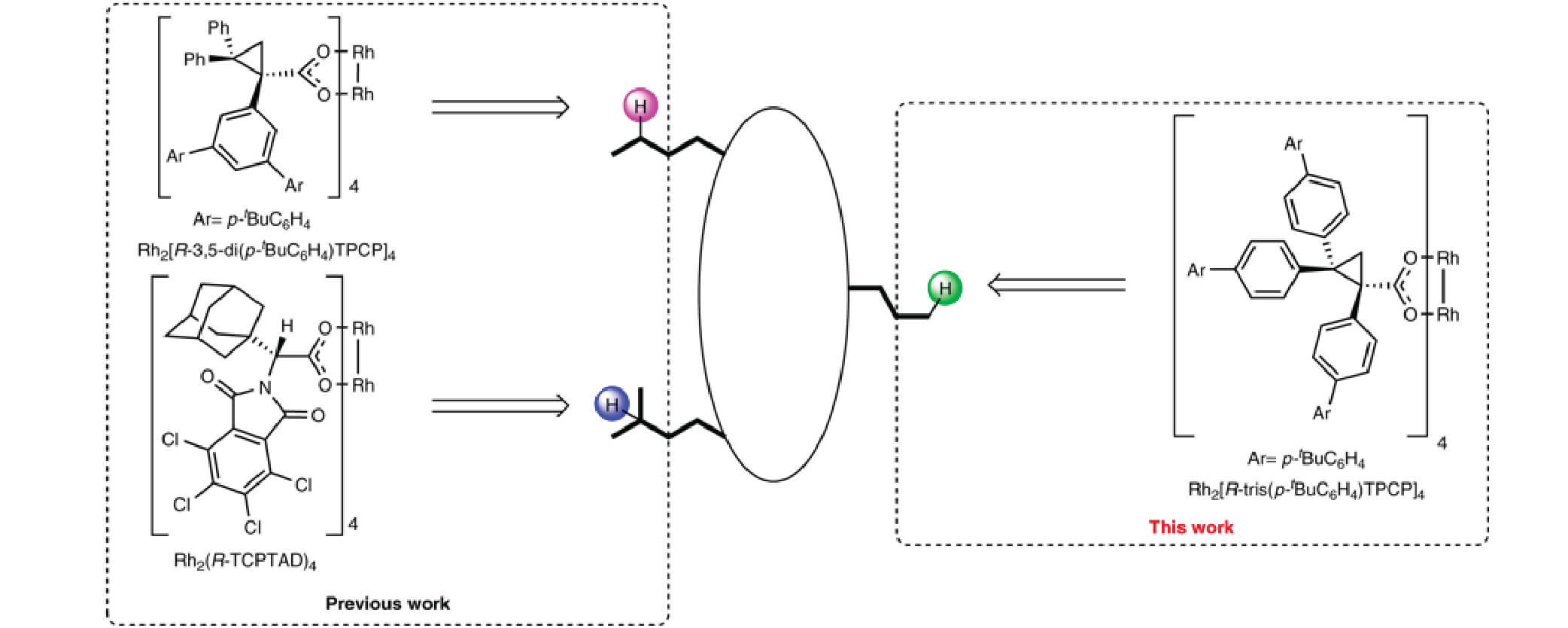
08/2018
Design of catalysts for site-selective and enantioselective functionalization of non-activated primary C–H bonds
RESEARCH
-
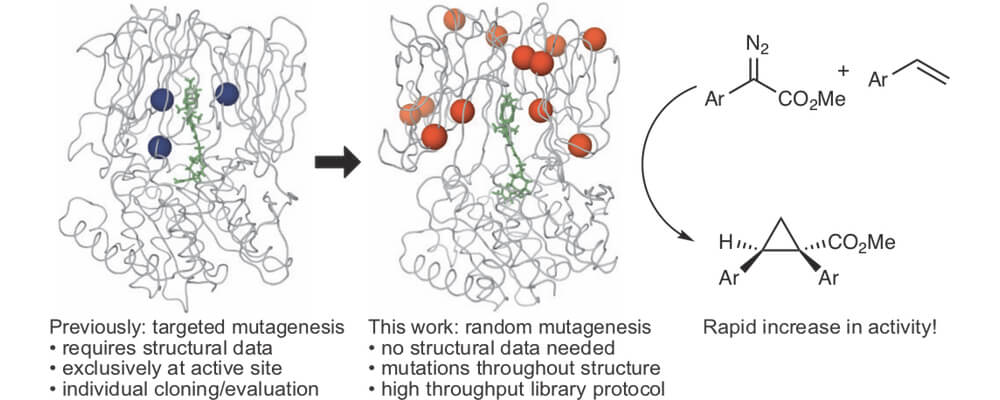
01/2018
Evolving artificial metalloenzymes via random mutagenesis
RESEARCH
-
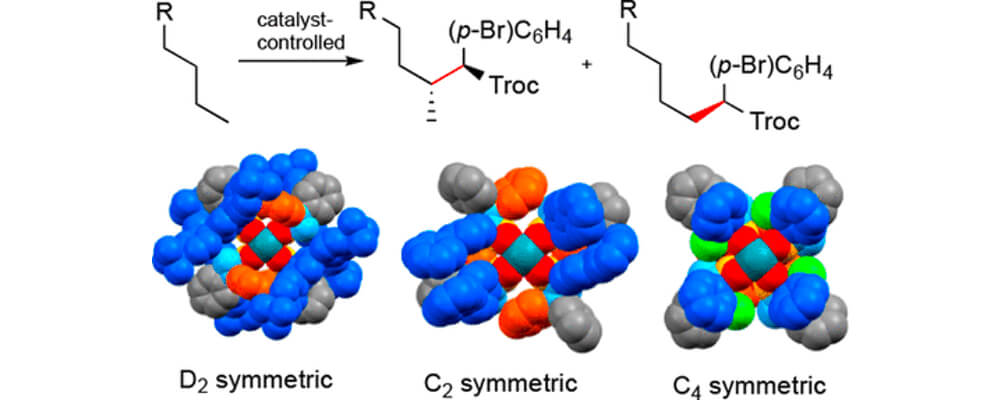
01/2018
Site-Selective Carbene-Induced C–H Functionalization Catalyzed by Dirhodium Tetrakis(triaryl cyclopropanecarboxylate) Complexes
RESEARCH
-
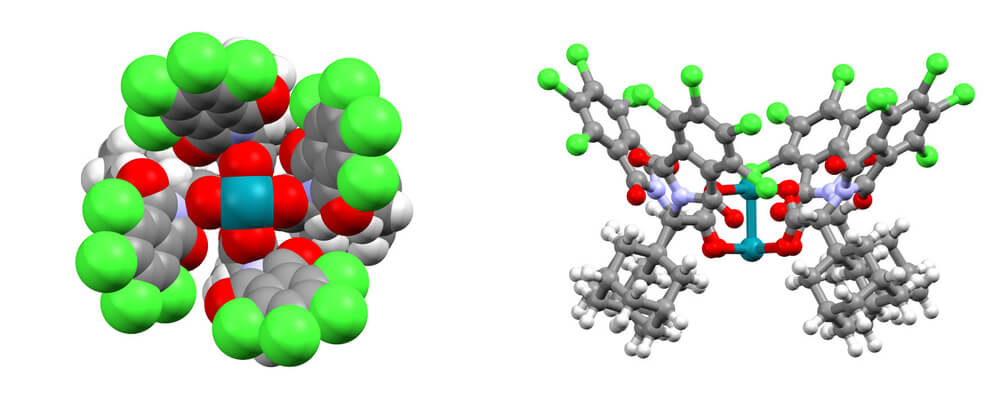
11/2017
Site-selective and stereoselective functionalization of non-activated tertiary C–H bonds
RESEARCH
-
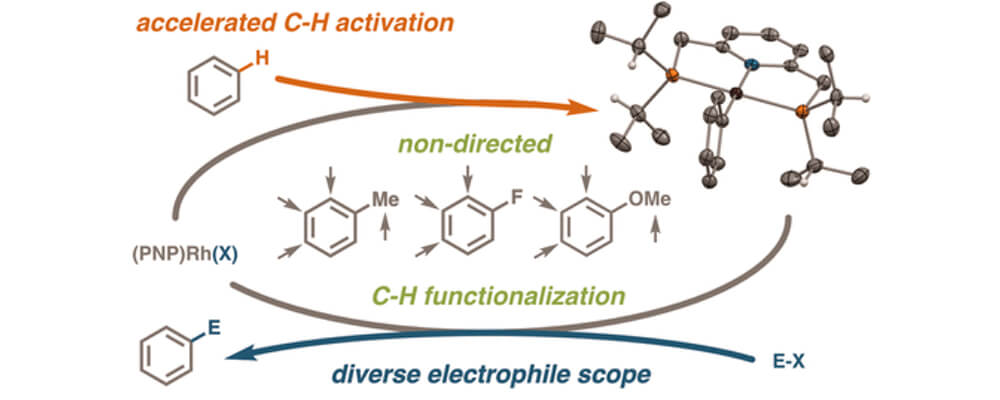
11/2017
Rhodium Complexes of 2,6-Bis(dialkylphosphinomethyl)pyridines: Improved C–H Activation, Expanded Reaction Scope, and Catalytic Direct Arylation
RESEARCH
-
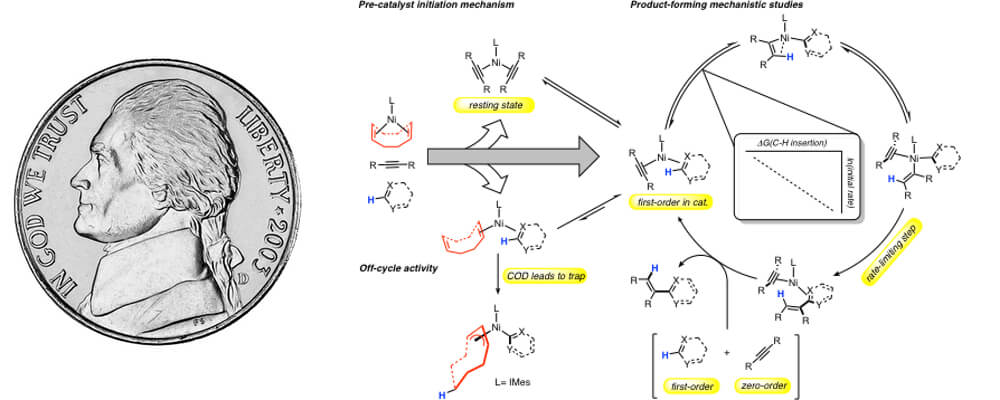
09/2017
Entrances, Traps, and Rate-Controlling Factors for Nickel-Catalyzed C−H Functionalization
RESEARCH
-
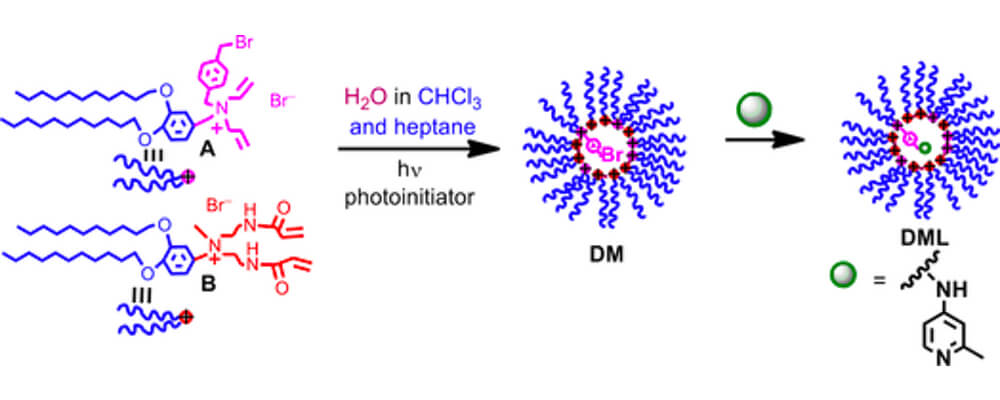
09/2017
Selective C(sp3)–H Monoarylation Catalyzed by a Covalently Cross-Linked Reverse Micelle-Supported Palladium Catalyst
RESEARCH
-
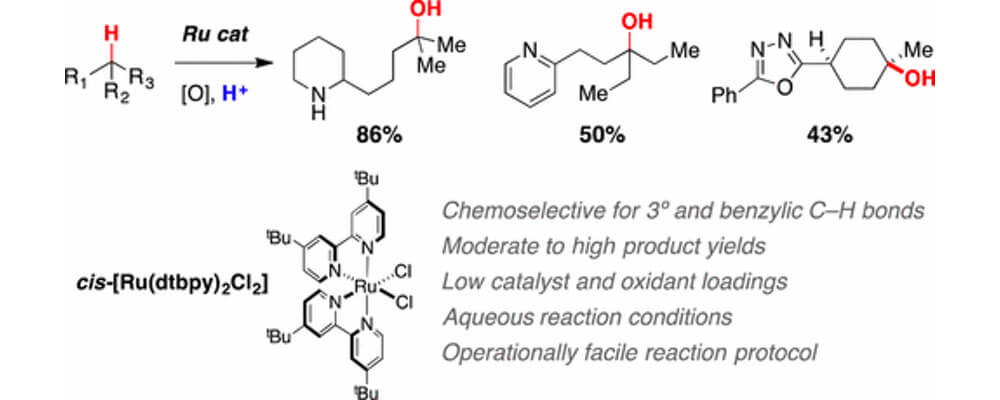
06/2017
Ruthenium-Catalyzed C–H Hydroxylation in Aqueous Acid Enables Selective Functionalization of Amine Derivatives
RESEARCH
-
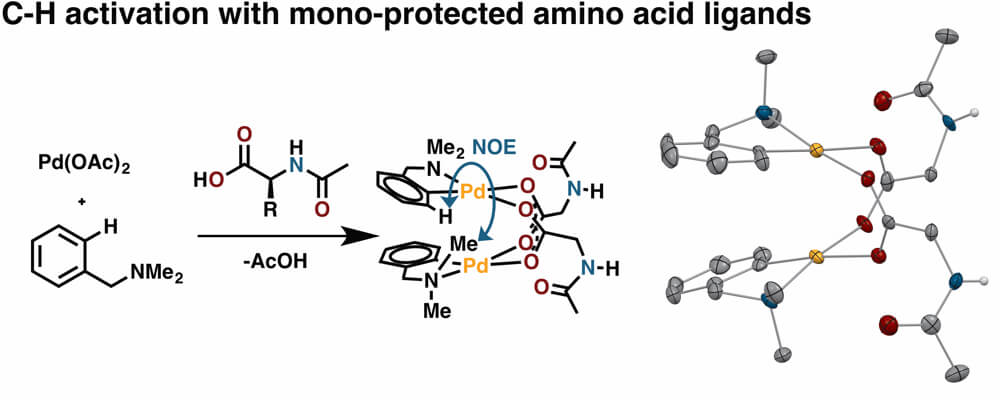
06/2017
Mono-N-protected amino acid ligands stabilize dimeric palladium(II) complexes of importance to C–H functionalization
RESEARCH
-
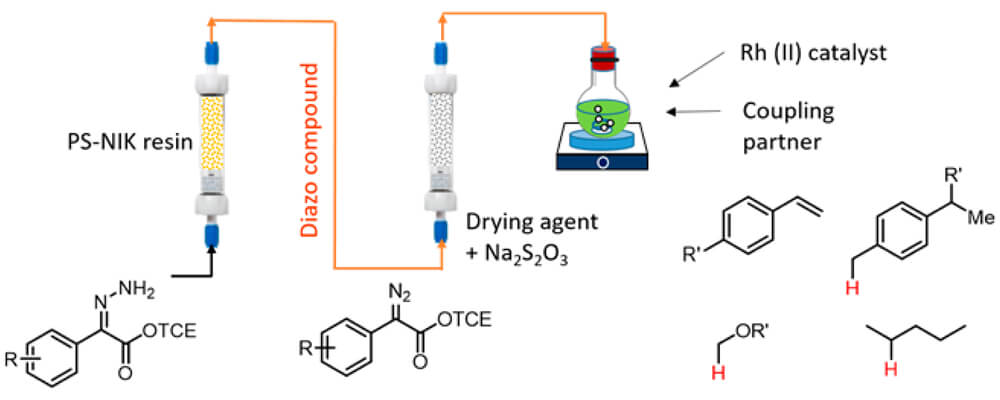
06/2017
Synthesis of D/A-Substituted Diazo Compounds in Flow and Their Application in Enantioselective Dirhodium-Catalyzed Cyclopropanation and C–H Functionalization
RESEARCH
-
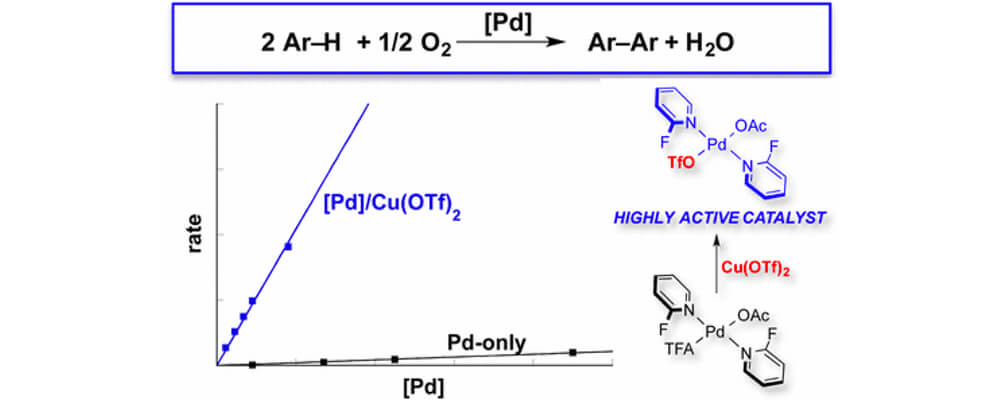
04/2017
Pd-Catalyzed Aerobic Oxidative Biaryl Coupling: Non-Redox Cocatalysis by Cu(OTf)2 and Discovery of Fe(OTf)3 as a Highly Effective Cocatalyst
RESEARCH
-
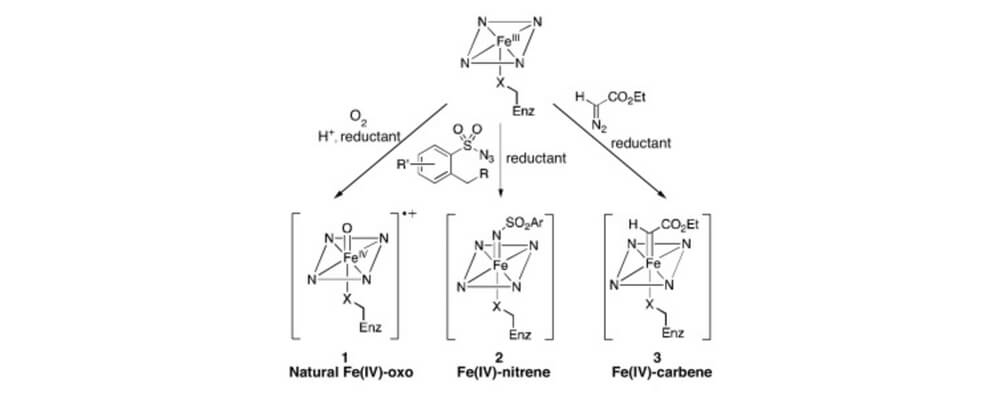
02/2017
Selective C–H bond functionalization using repurposed or artificial metalloenzymes
RESEARCH
-
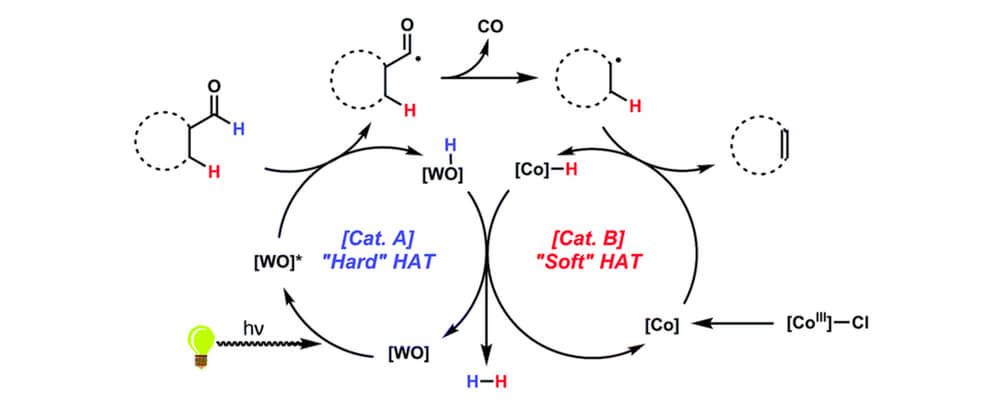
11/2016
Toward a mild dehydroformylation using base-metal catalysis
RESEARCH
-
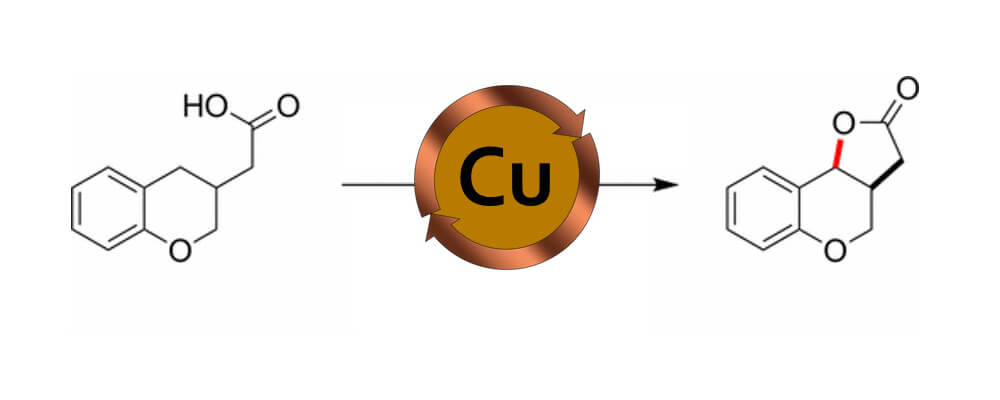
012/2016
Copper-Catalyzed Oxidative Cyclization of Carboxylic Acids
RESEARCH
-
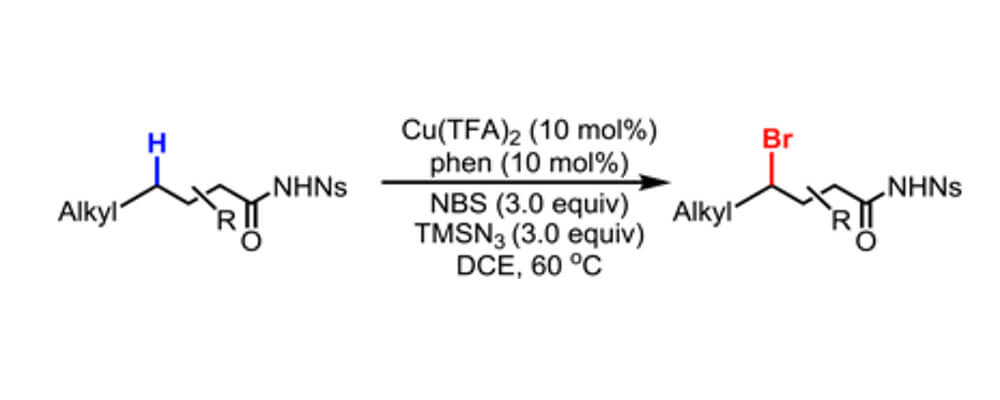
11/2016
Copper-Catalyzed Bromination of C(sp3)−H Bonds Distal to Functional Groups
RESEARCH
-
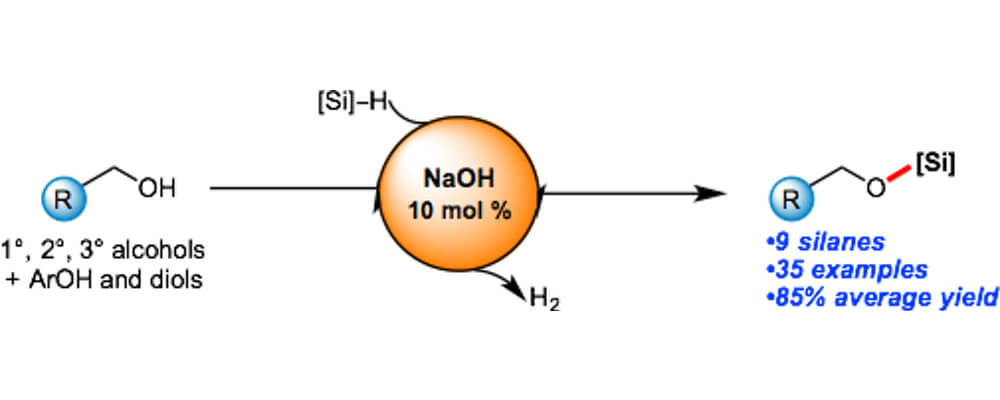
11/2016
Sodium Hydroxide Catalyzed Dehydrocoupling of Alcohols with Hydrosilanes
RESEARCH
-
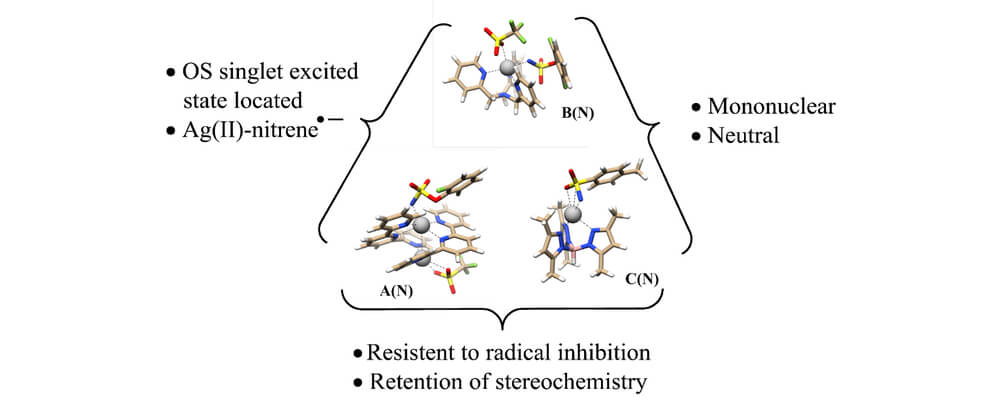
10/2016
Catalyst-Controlled and Tunable, Chemoselective Silver-Catalyzed Intermolecular Nitrene Transfer: Experimental and Computational Studies
RESEARCH
-
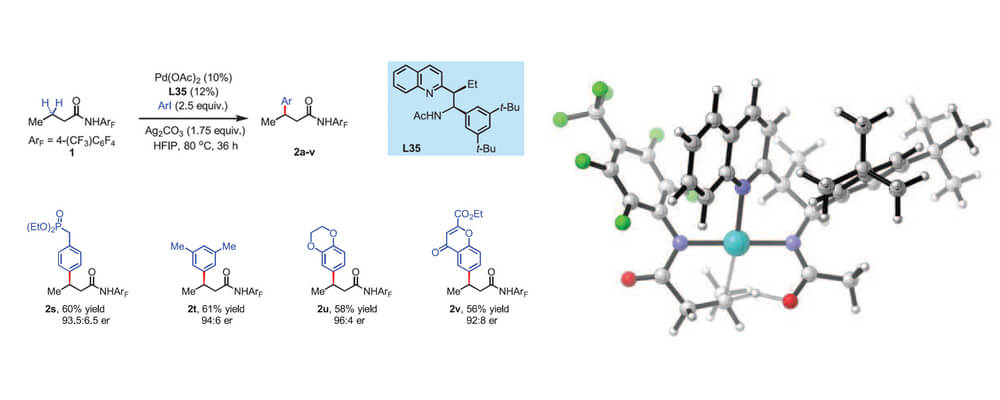
09/2016
Ligand-accelerated enantioselective methylene C(sp3)–H bond activation
RESEARCH
-

07/2016
Biochemical and Structural Characterization of MycCI, a Versatile P450 Biocatalyst from the Mycinamicin Biosynthetic Pathway
RESEARCH
-
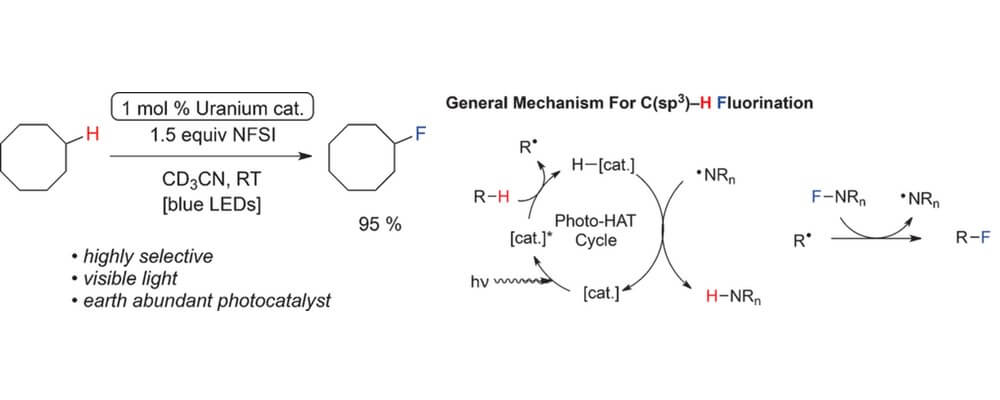
06/2016
The Uranyl Cation as a Visible-Light Photocatalyst for C(sp3)−H Fluorination
RESEARCH
-
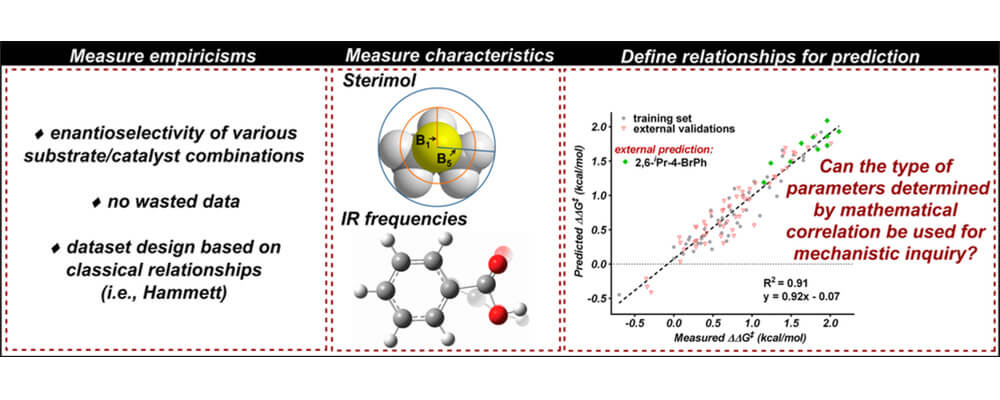
05/2016
The Development of Multidimensional Analysis Tools for Asymmetric Catalysis and Beyond
RESEARCH
-
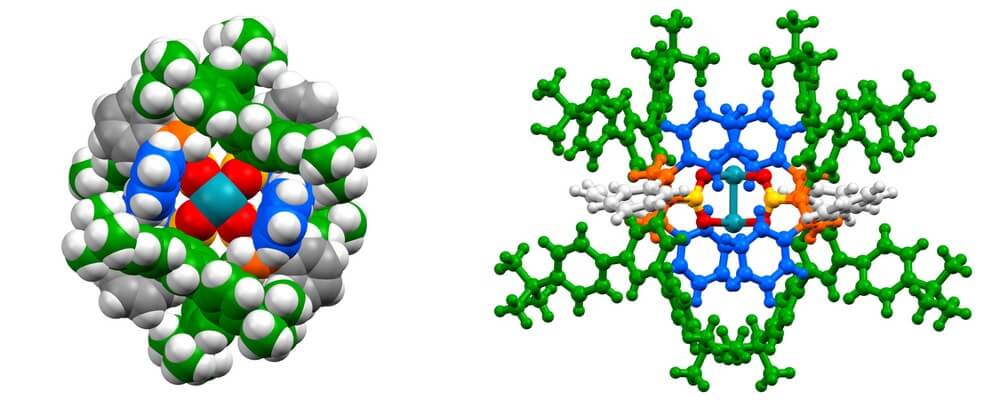
05/2016
Site Selective and Stereoselective Functionalization of Unactivated C–H Bonds
RESEARCH
-
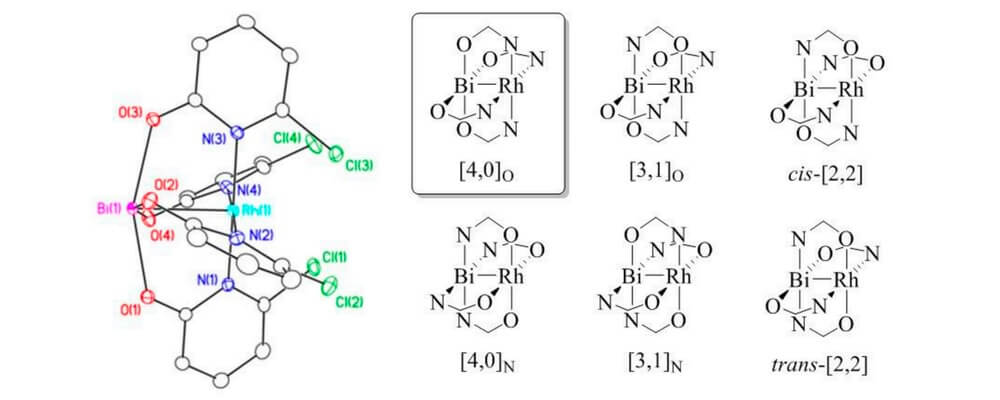
05/2016
The first bismuth(II)–rhodium(II) oxypyridinate paddlewheel complexes: Synthesis and structural characterization
RESEARCH
-
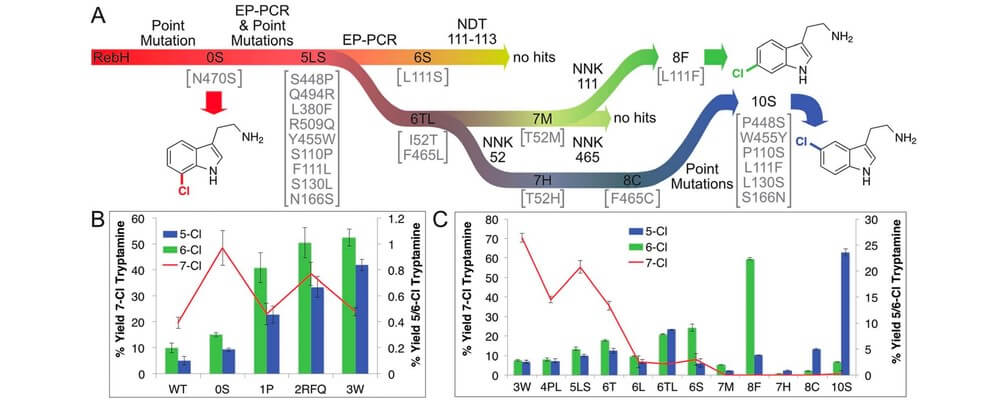
02/2016
Directed evolution of RebH for catalyst-controlled halogenation of indole C–H bonds
RESEARCH
-
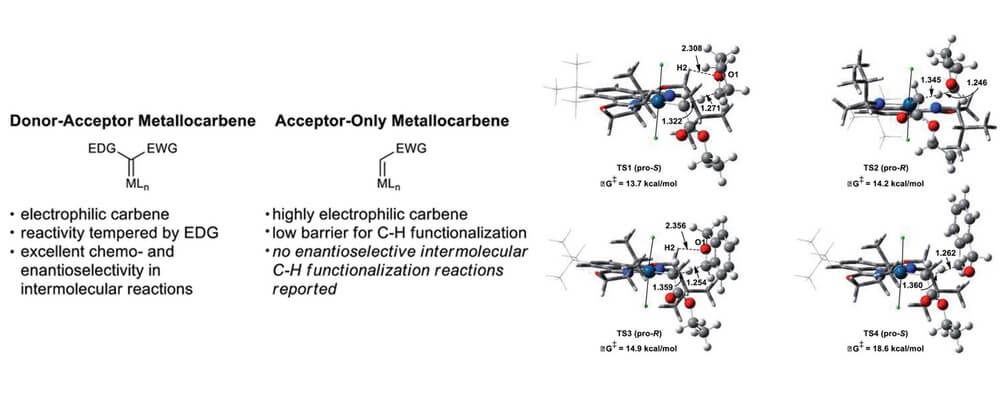
02/2016
Iridium(III)-bis(imidazolinyl)phenyl catalysts for enantioselective C–H functionalization with ethyl diazoacetate
RESEARCH
-
01/2016
Rh2(II,III) Catalysts with Chelating Carboxylate and Carboxamidate Supports: Electronic Structure and Nitrene Transfer Reactivity
RESEARCH
-
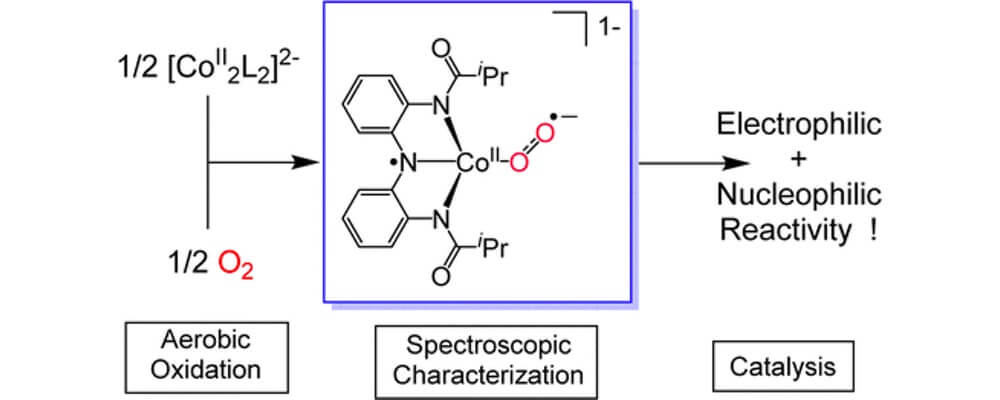
01/2016
Oxygen Activation by Co(II) and a Redox Non-Innocent Ligand: Spectroscopic Characterization of a Radical–Co(II)–Superoxide Complex with Divergent Catalytic Reactivity
RESEARCH
-
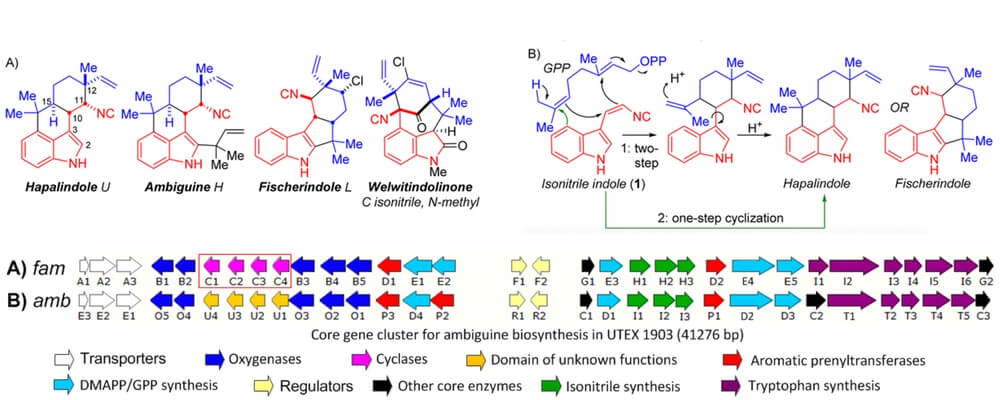
12/2015
Hapalindole/Ambiguine Biogenesis Is Mediated by a Cope Rearrangement, C–C Bond-Forming Cascade
RESEARCH
-
12/2015
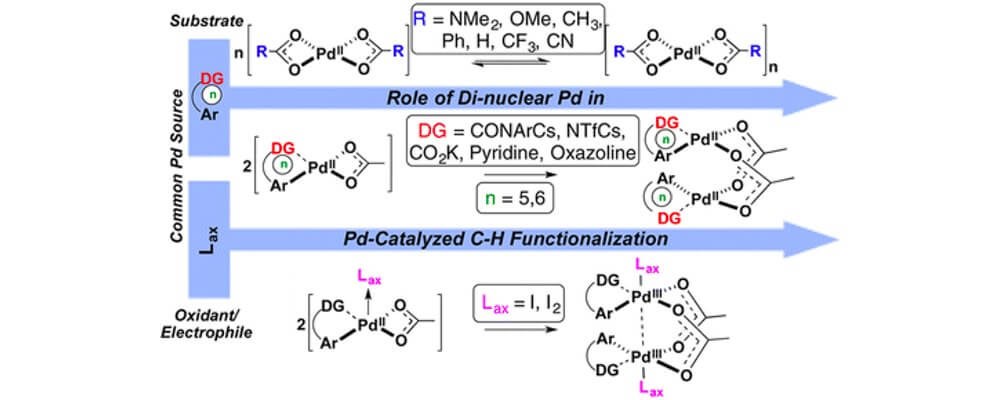
Factors Controlling Stability and Reactivity of Dimeric Pd(II) Complexes in C–H Functionalization Catalysis
RESEARCH
-
12/2015
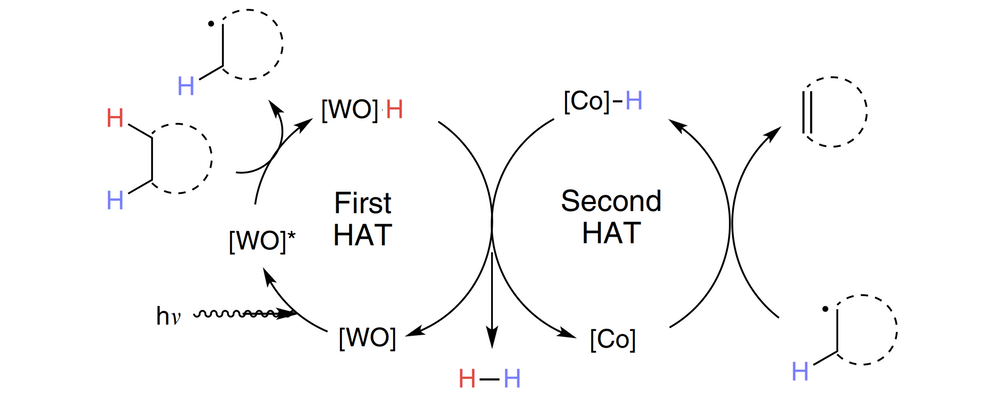
Acceptorless dehydrogenation of small molecules through cooperative base metal catalysis
RESEARCH
-
11/2015
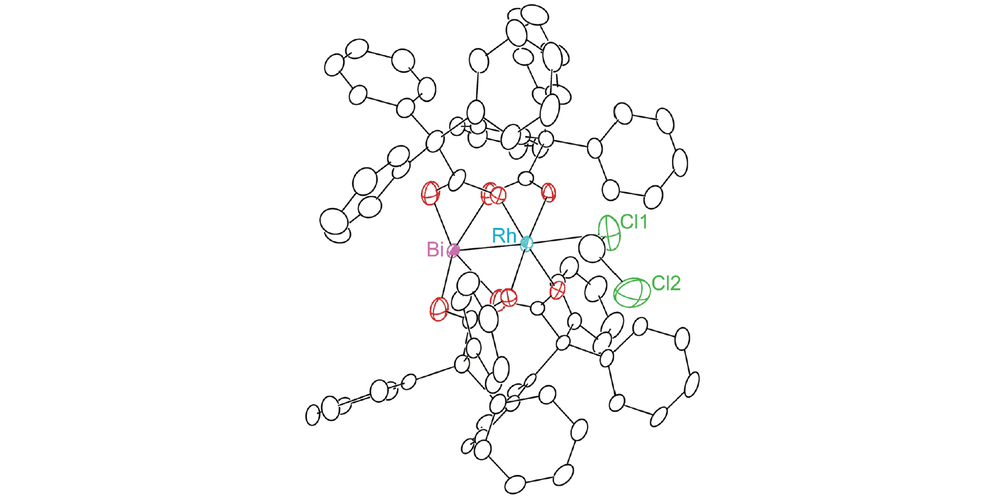
Expanding the family of heterobimetallic Bi–Rh paddlewheel carboxylate complexes via equatorial carboxylate exchange
RESEARCH
-
12/2015
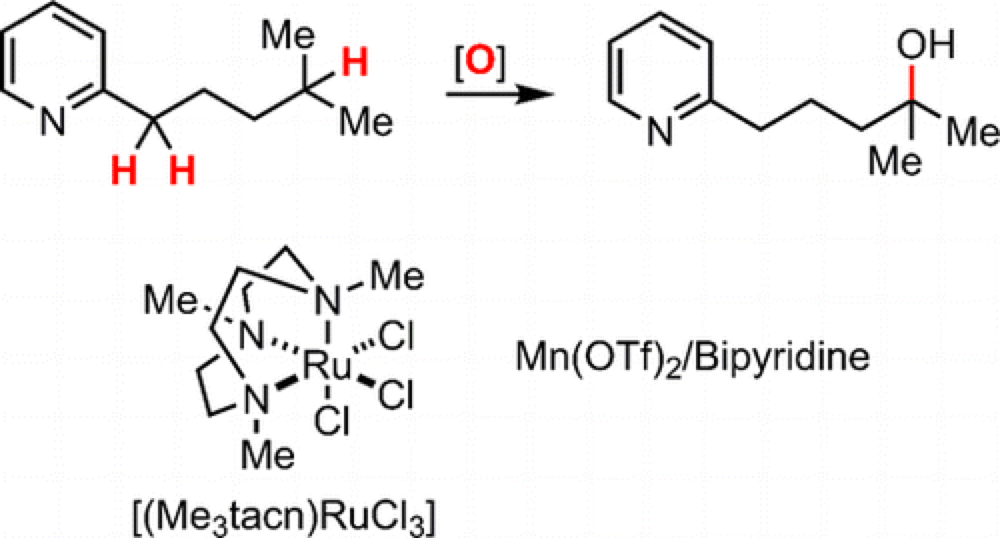
Comparative Study of the Limitations and Challenges in Atom-Transfer C–H Oxidations
RESEARCH
-
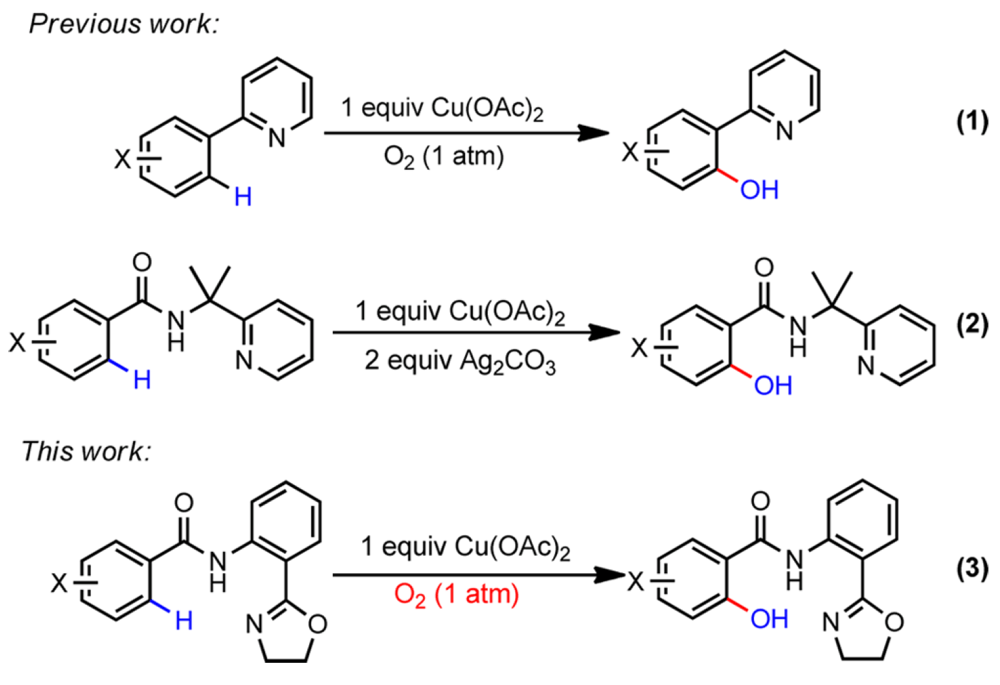
08/2015
Cu(II)-Mediated C(sp2)–H Hydroxylation
RESEARCH
-
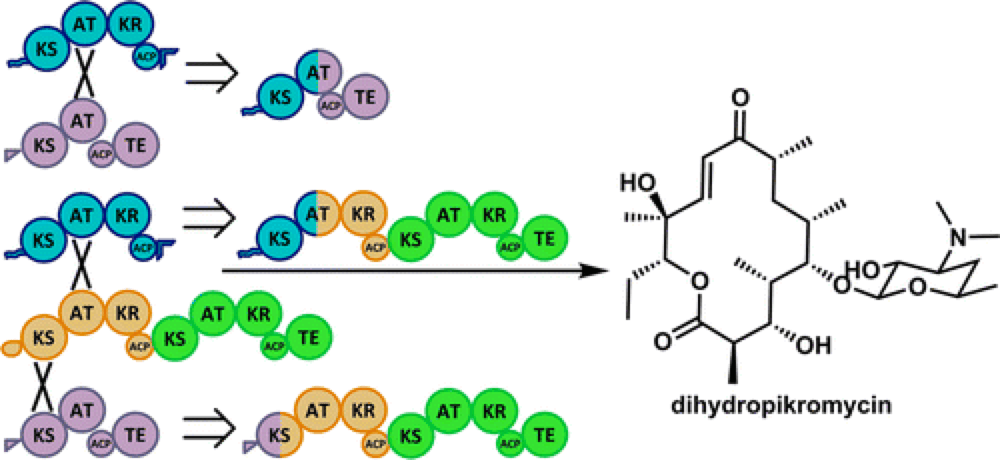
07/2015
Evolution of Efficient Modular Polyketide Synthases by Homologous Recombination
RESEARCH
-
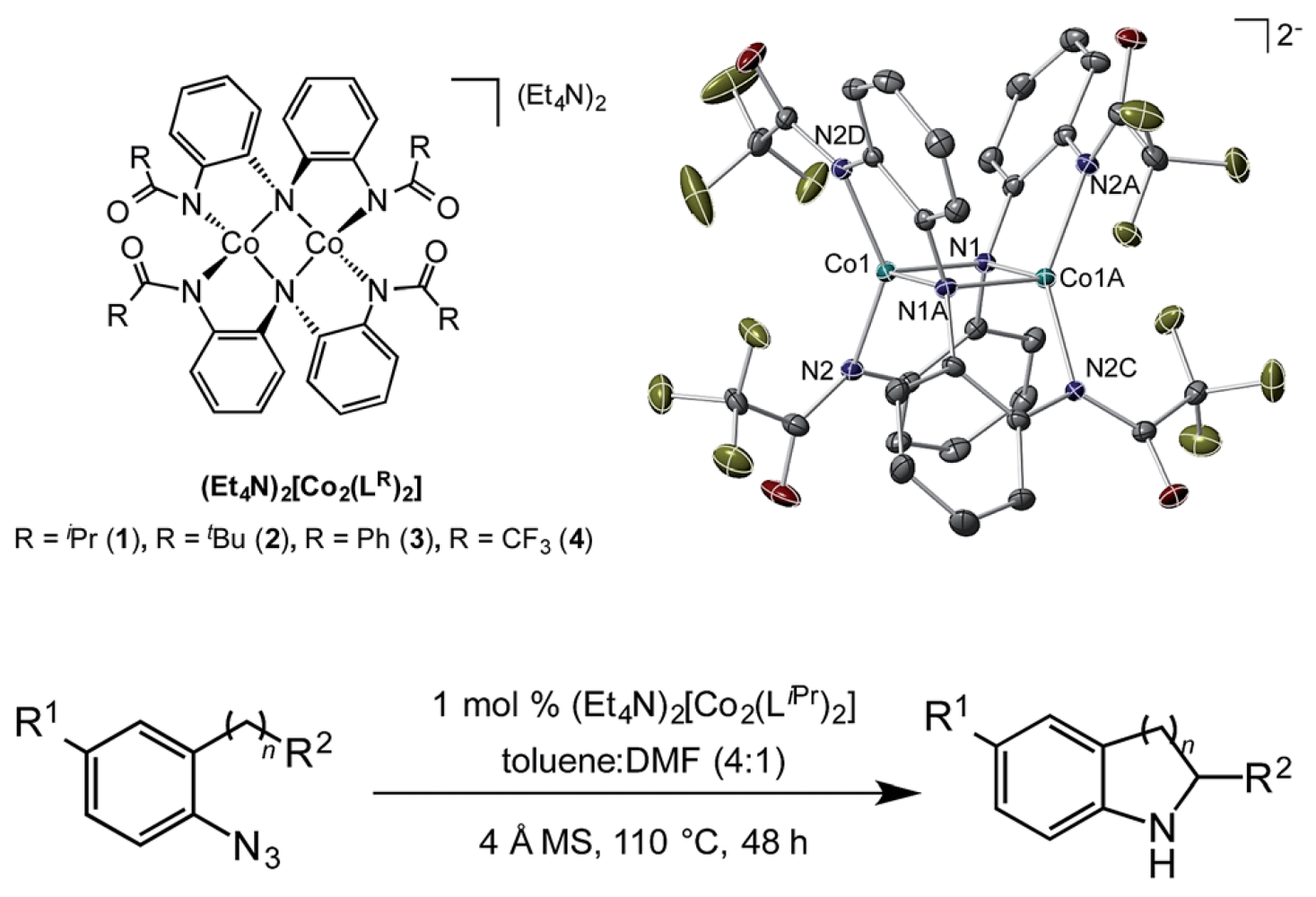
07/2015
Cobalt catalyzed sp3 C–H amination utilizing aryl azides
RESEARCH
-
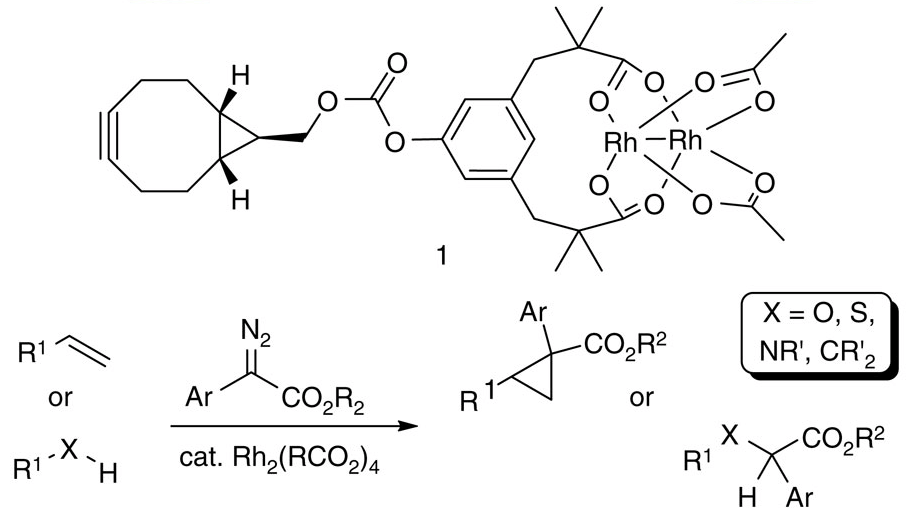
07/2015
Engineering a dirhodium artificial metalloenzyme for selective olefin cyclopropanation
RESEARCH
-
06/2015
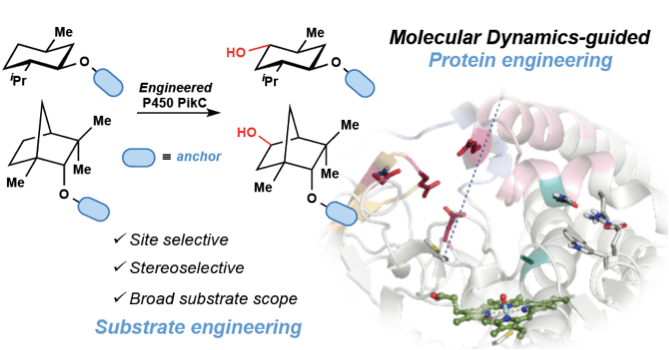
Enzymatic hydroxylation of an unactivated methylene C–H bond guided by molecular dynamics simulations
RESEARCH
-
06/2015
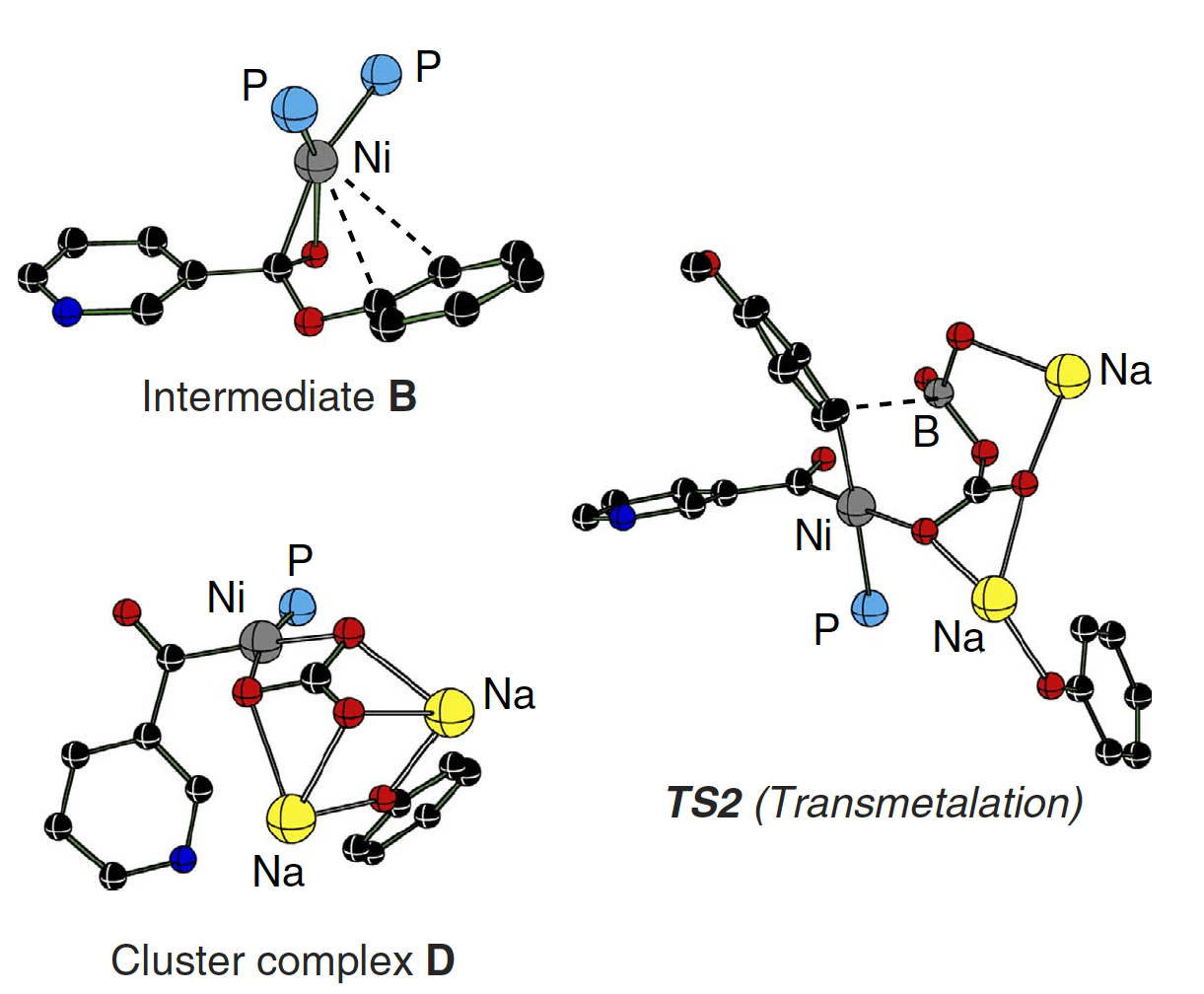
Decarbonylative cross-coupling of esters by Ni-catalysis
RESEARCH
-
06/2015
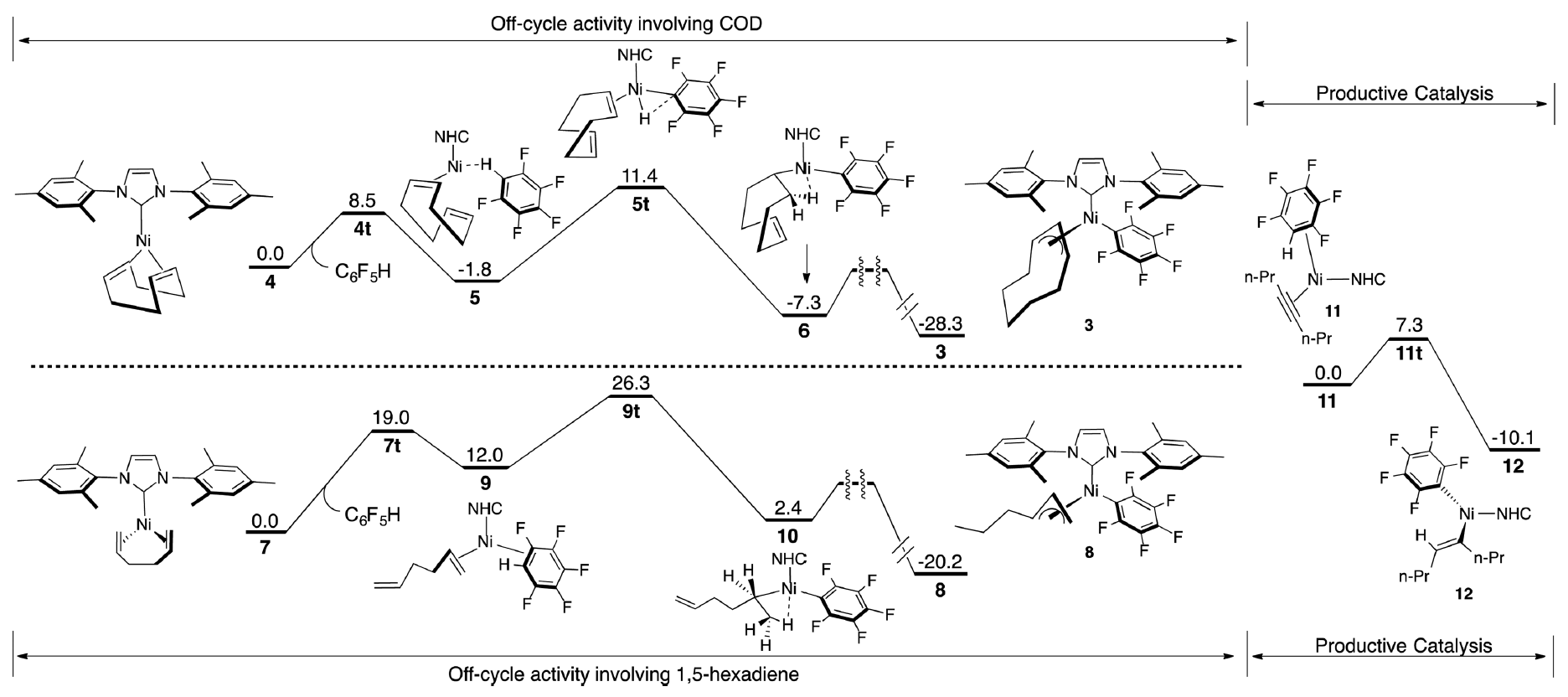
Highly Active Nickel Catalysts for C–H Functionalization Identified through Analysis of Off-Cycle Intermediates
RESEARCH
-
06/2015
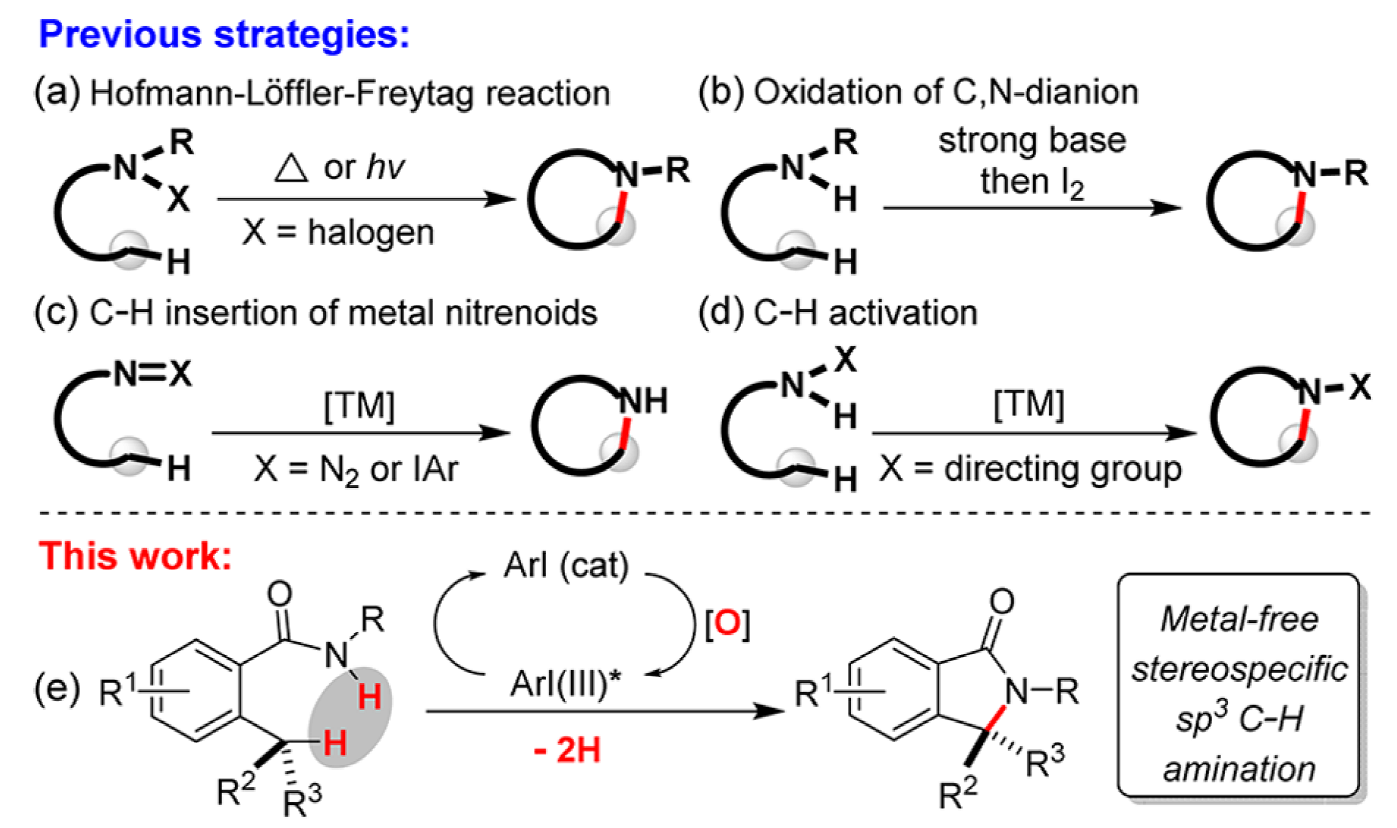
Iodoarene-Catalyzed Stereospecific Intramolecular sp3 C–H Amination
RESEARCH
-
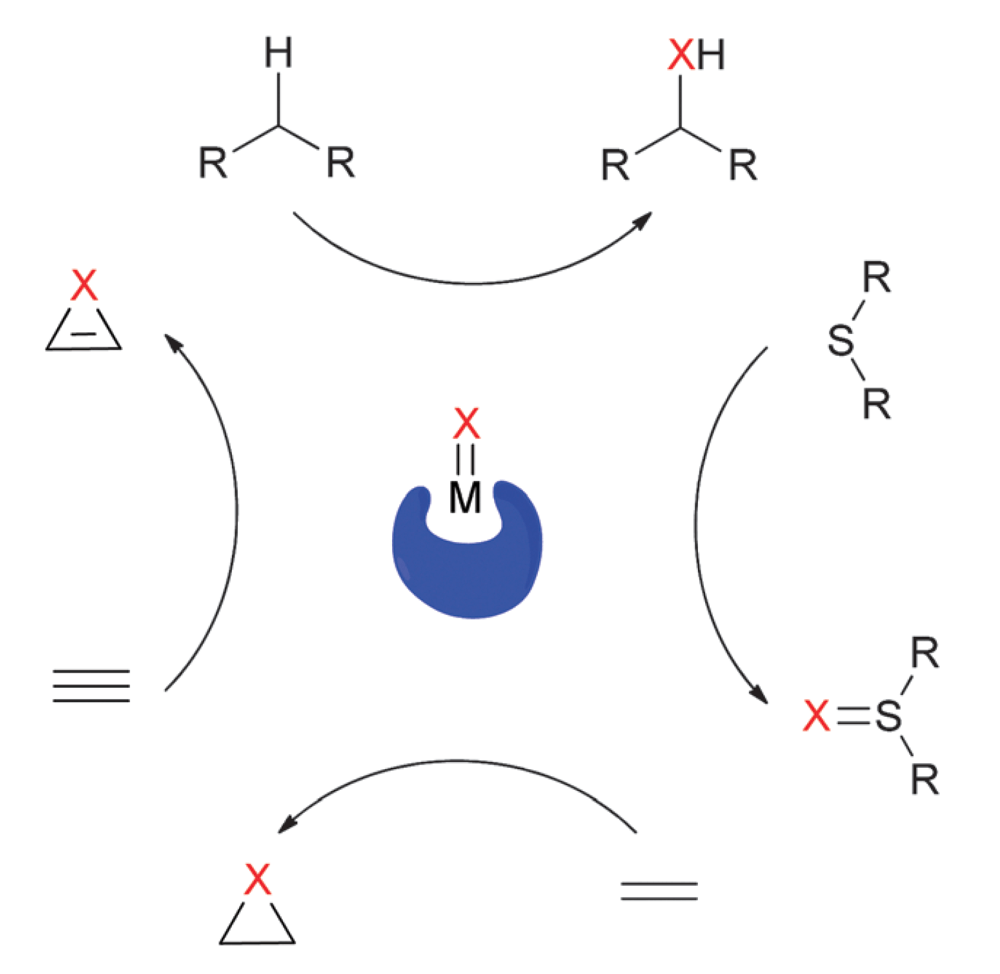
06/2015
Ir-Catalyzed enantioselective group transfer reactions
RESEARCH
-
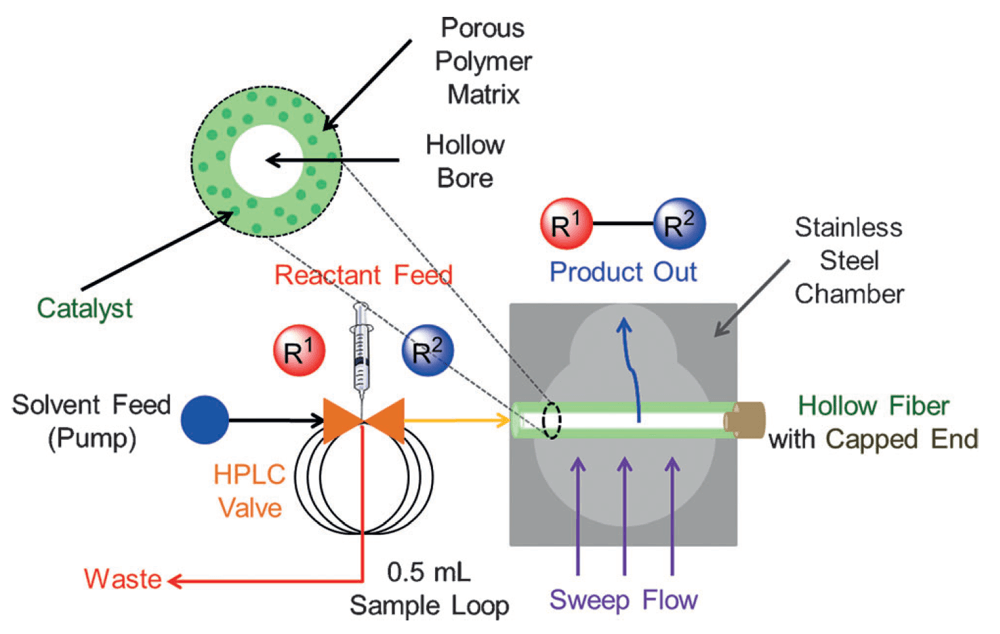
04/2015
Composite Polymer/Oxide Hollow Fiber Contactors: Versatile and Scalable Flow Reactors
RESEARCH
-
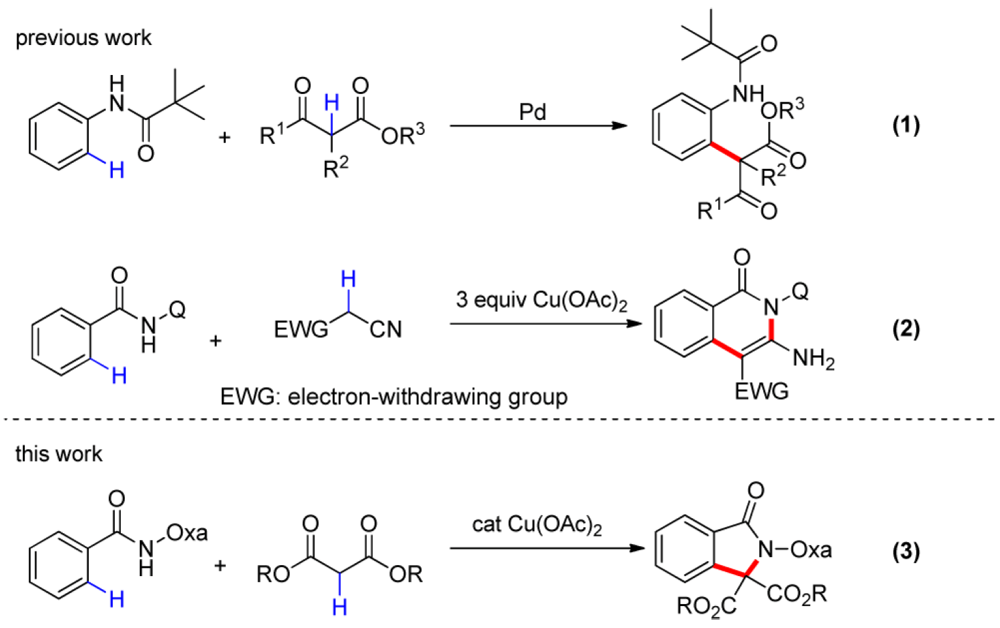
02/2015
Cu(II)-Catalyzed Coupling of Aromatic C–H Bonds with Malonates
RESEARCH
-
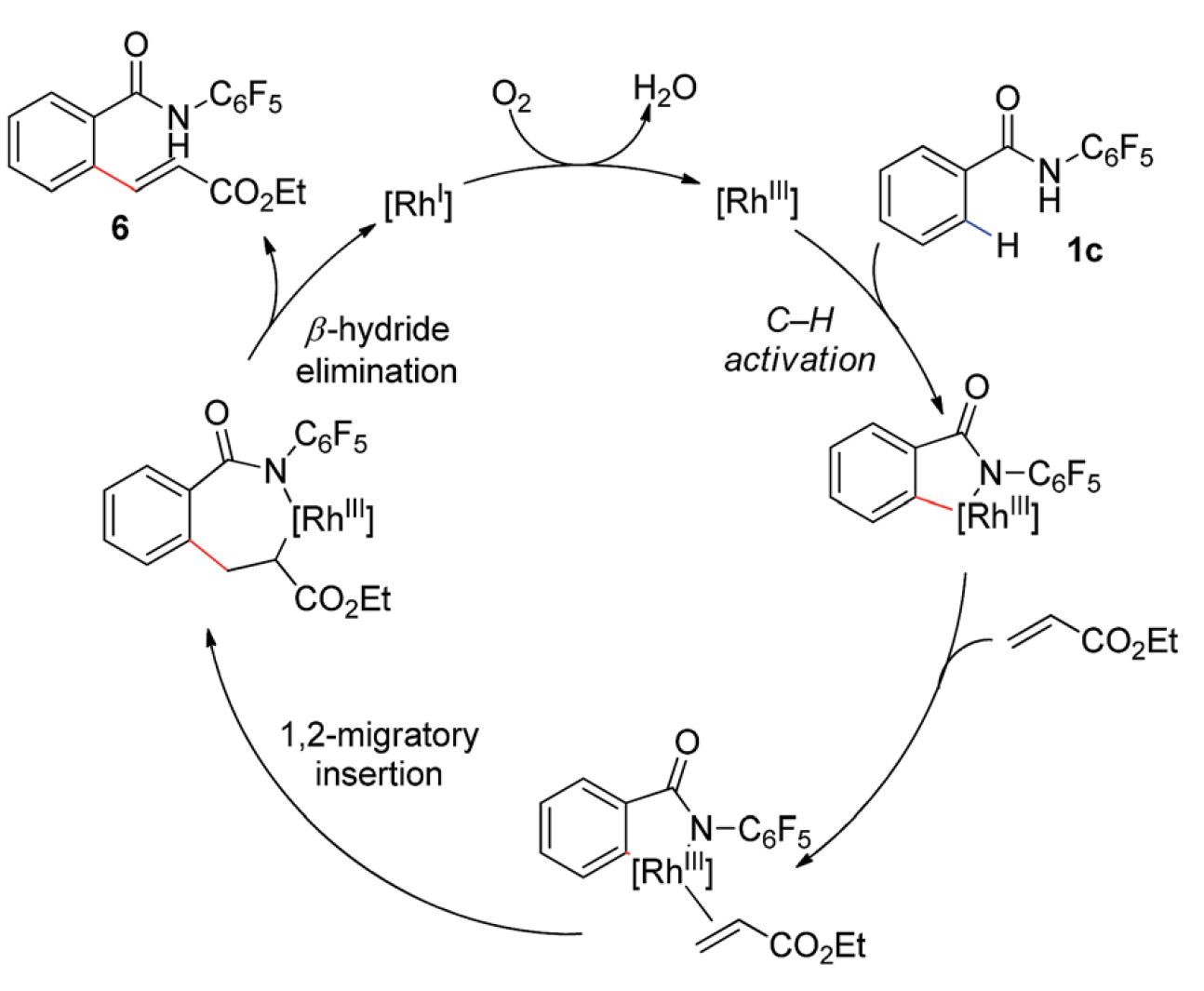
01/2015
Rh(III)-catalyzed C–H olefination of N-pentafluoroaryl benzamides
RESEARCH
-
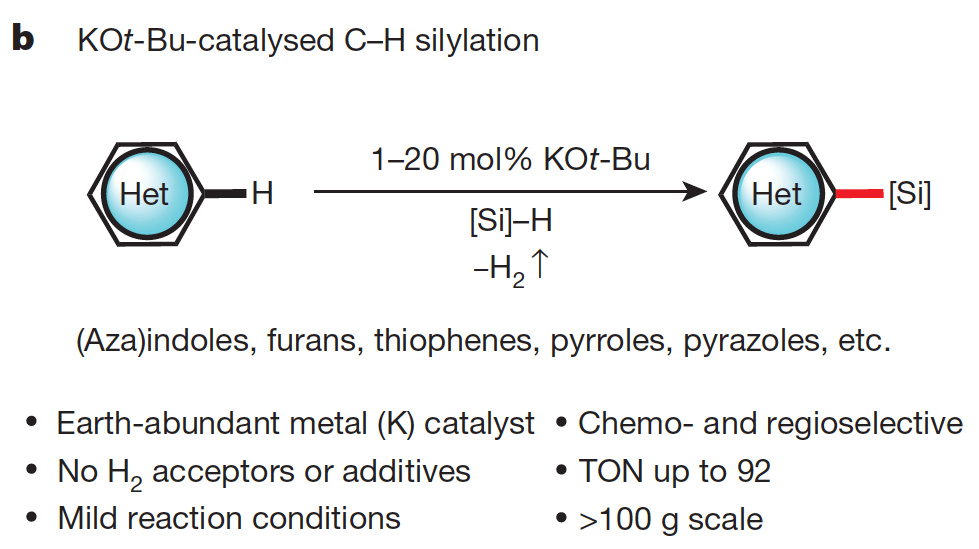
01/2015
Silylation of C–H bonds in aromatic heterocycles by an Earth-abundant metal catalyst
RESEARCH
-
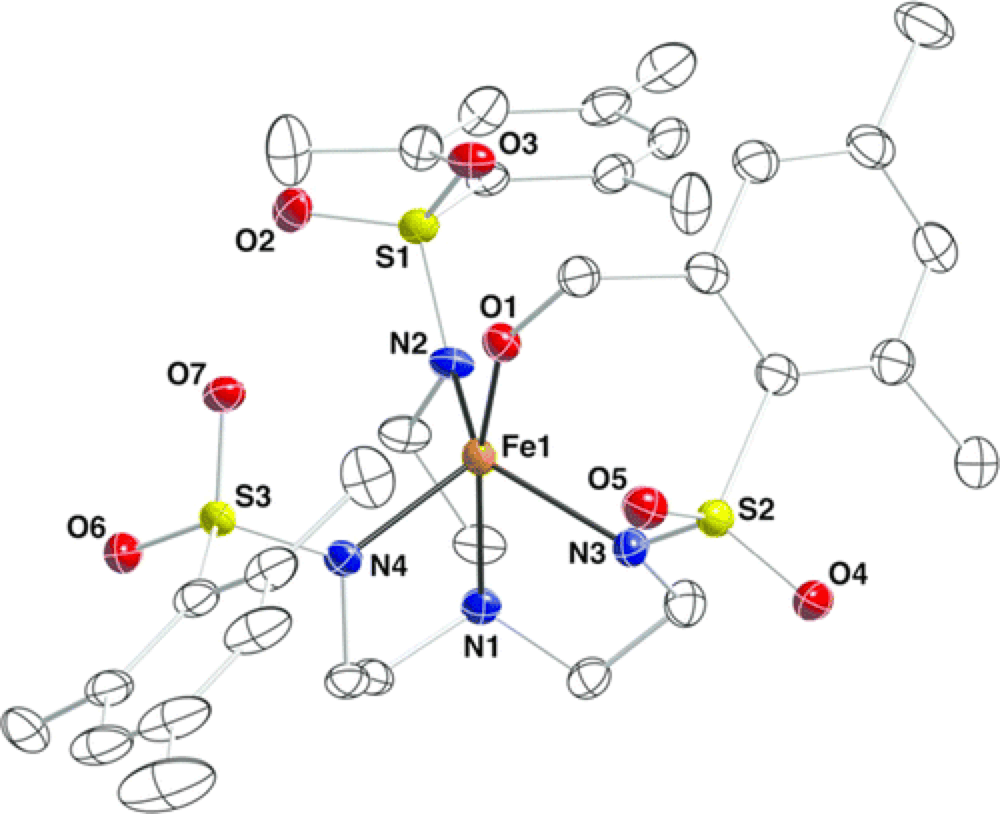
09/2014
Iron(II) Complexes Supported by Sulfonamido Tripodal Ligands
RESEARCH
-
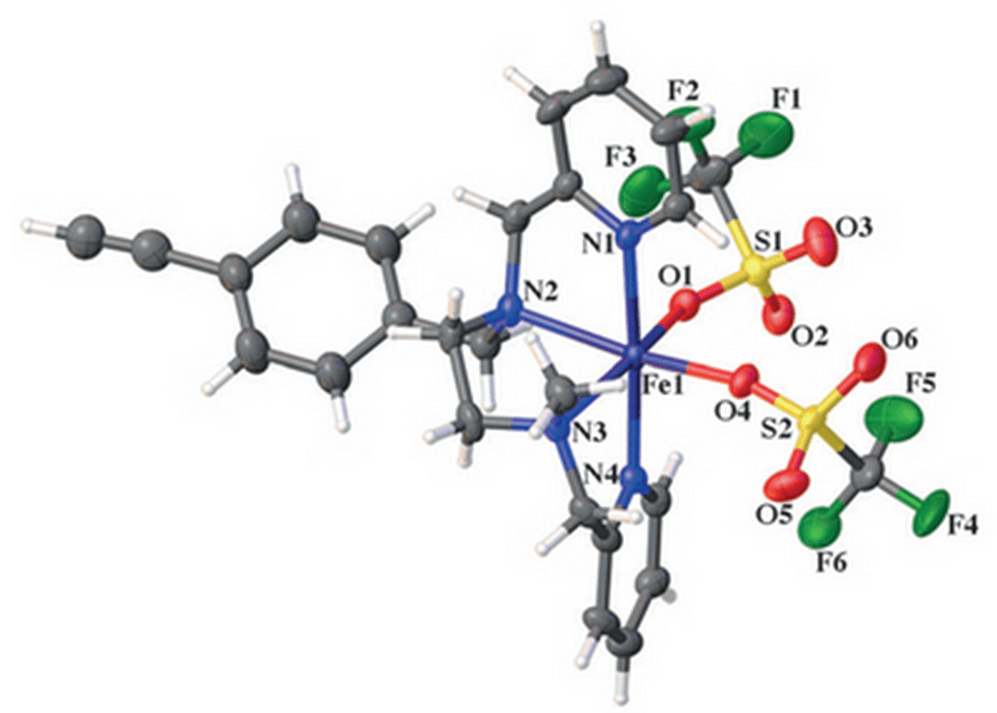
10/2014
Polymer- and Silica-Supported Iron BPMEN-Inspired Catalysts for C–H Functionalization
RESEARCH
-
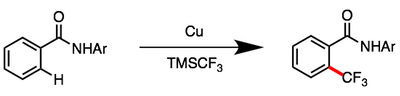
08/2014
Exceedingly Fast Copper(II)-Promoted ortho C–H Trifluoromethylation of Arenes using TMSCF3
RESEARCH
-
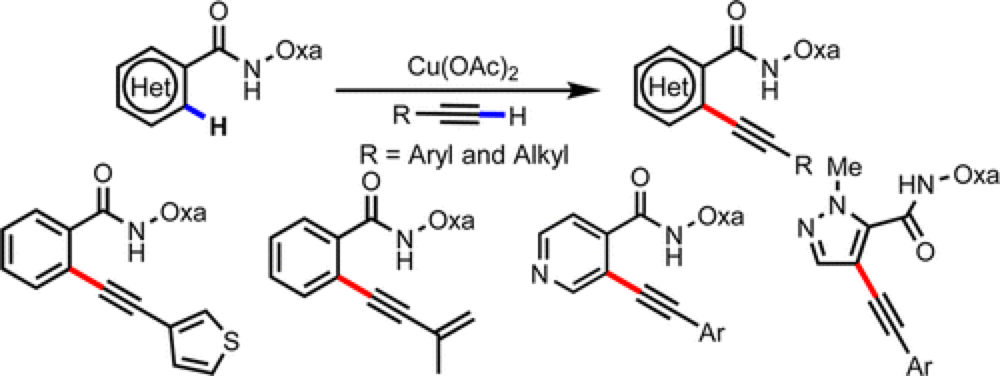
08/2014
Cu(II)-Mediated Ortho C–H Alkynylation of (Hetero)Arenes with Terminal Alkynes
RESEARCH
-
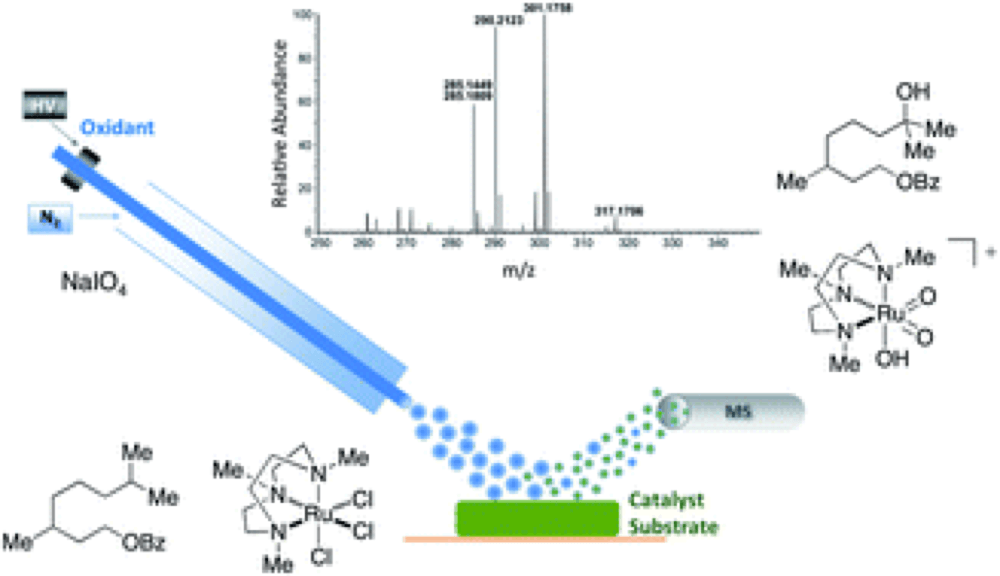
07/2014
Speciation and decomposition pathways of ruthenium catalysts used for selective C–H hydroxylation
RESEARCH
-
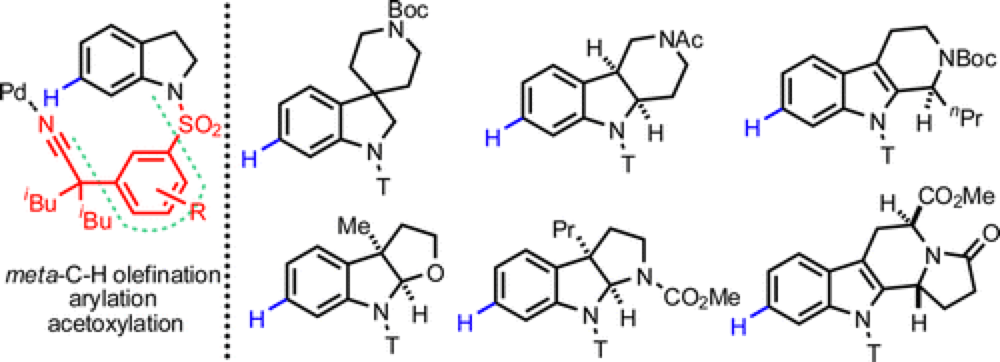
07/2014
Pd(II)-Catalyzed meta-C–H Olefination, Arylation, and Acetoxylation of Indolines Using a U-Shaped Template
RESEARCH
-
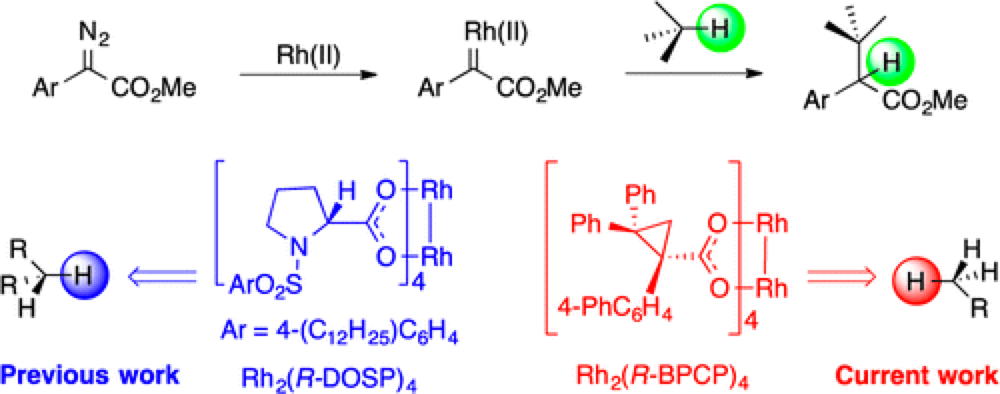
06/2014
Role of Sterically Demanding Chiral Dirhodium Catalysts in Site-Selective C–H Functionalization of Activated Primary C–H Bonds
RESEARCH
-
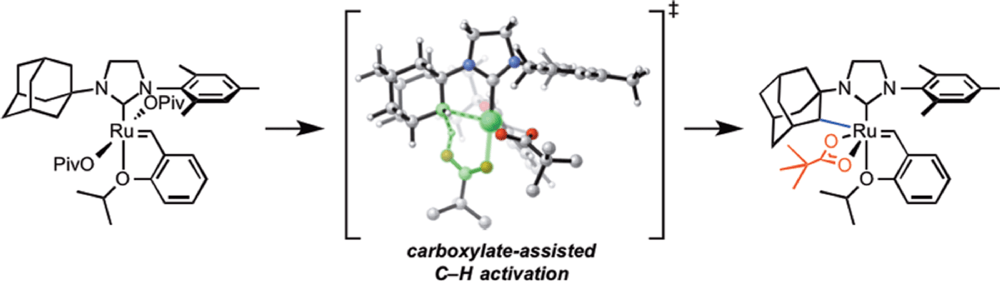
04/2014
Carboxylate-Assisted C(sp3)–H Activation in Olefin Metathesis-Relevant Ruthenium Complexes
RESEARCH
-
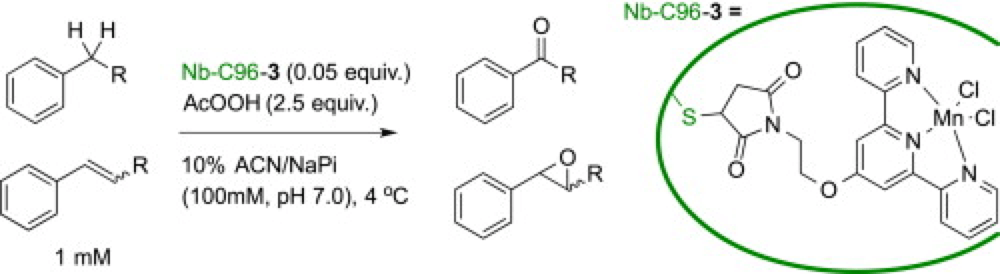
07/2014
Manganese terpyridine artificial metalloenzymes for benzylic oxygenation and olefin epoxidation
RESEARCH
-
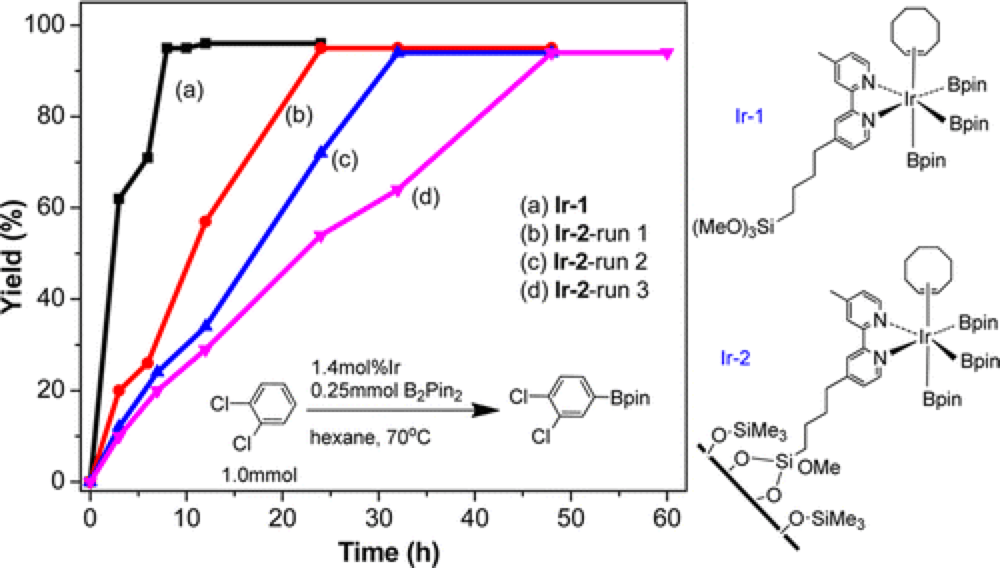
04/2014
Recyclable Silica-Supported Iridium Bipyridine Catalyst for Aromatic C–H Borylation
RESEARCH
-
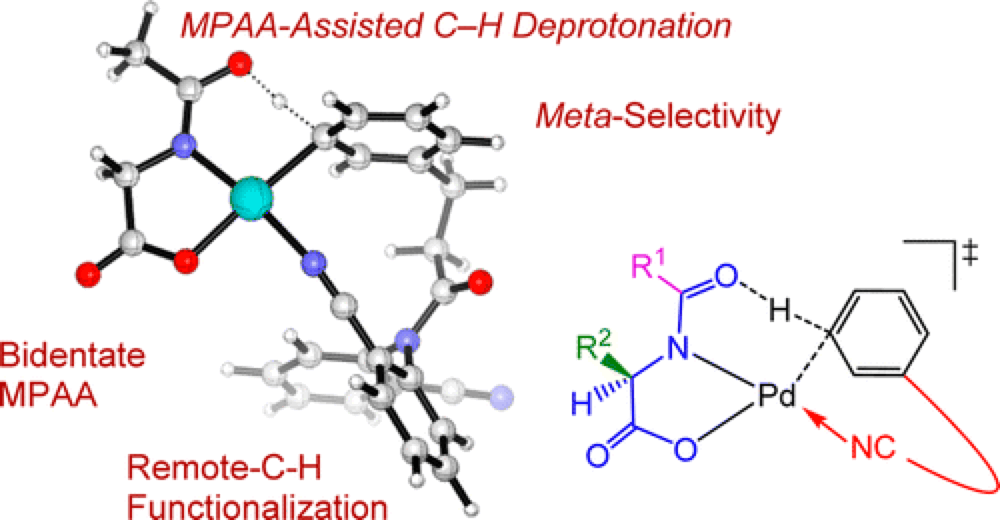
12/2013
Role of N-Acyl Amino Acid Ligands in Pd(II)-Catalyzed Remote C–H Activation of Tethered Arenes
RESEARCH
-
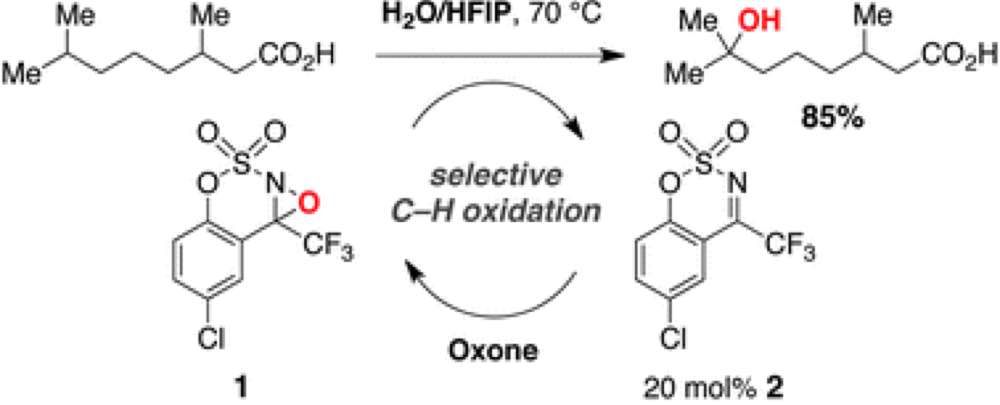
12/2013
Organocatalytic C–H hydroxylation with Oxone enabled by an aqueous fluoroalcohol solvent system
RESEARCH
-
12/2013
A General Method for Artificial Metalloenzyme Formation through Strain-Promoted Azide–Alkyne Cycloaddition
RESEARCH
-
11/2013
Silica-Immobilized Chiral Dirhodium(II) Catalyst for Enantioselective Carbenoid Reactions
RESEARCH
-
10/2013
Artificial Metalloenzymes and Metallopeptide Catalysts for Organic Synthesis
RESEARCH
-
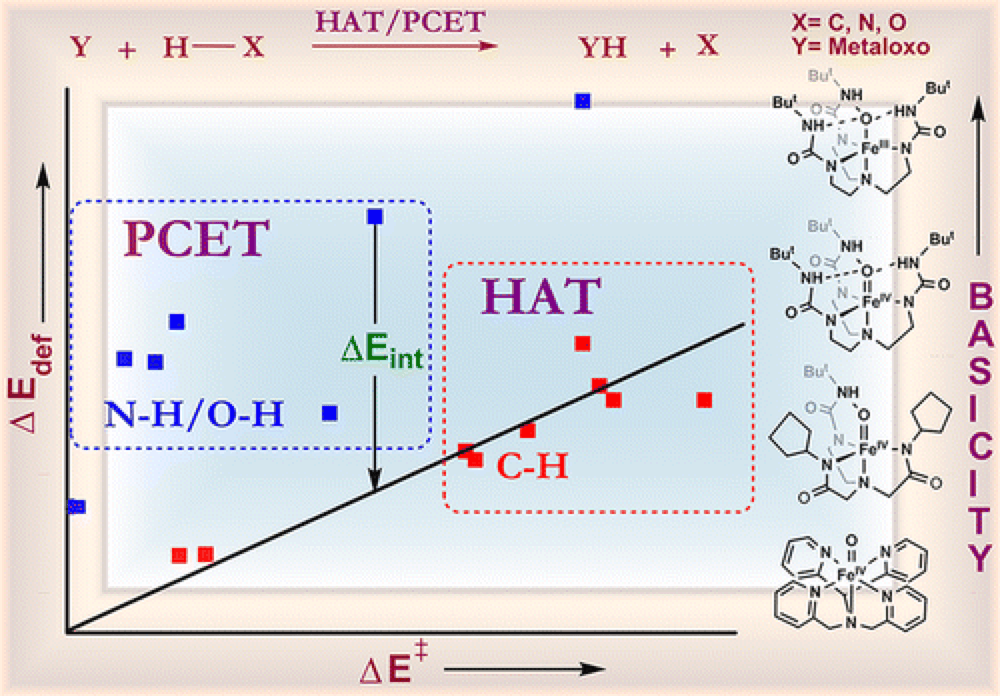
09/2013
Dichotomous Hydrogen Atom Transfer vs Proton-Coupled Electron Transfer
RESEARCH
-
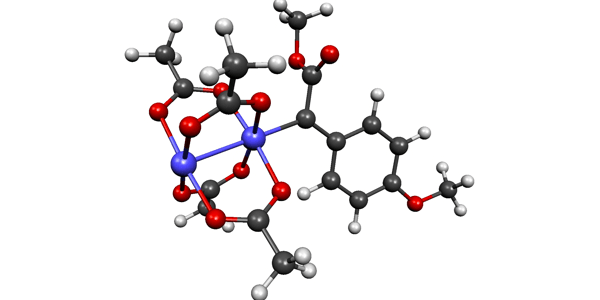
09/2013
Direct Spectroscopic Characterization of a Transitory Dirhodium Donor-Acceptor Carbene Complex
RESEARCH
-
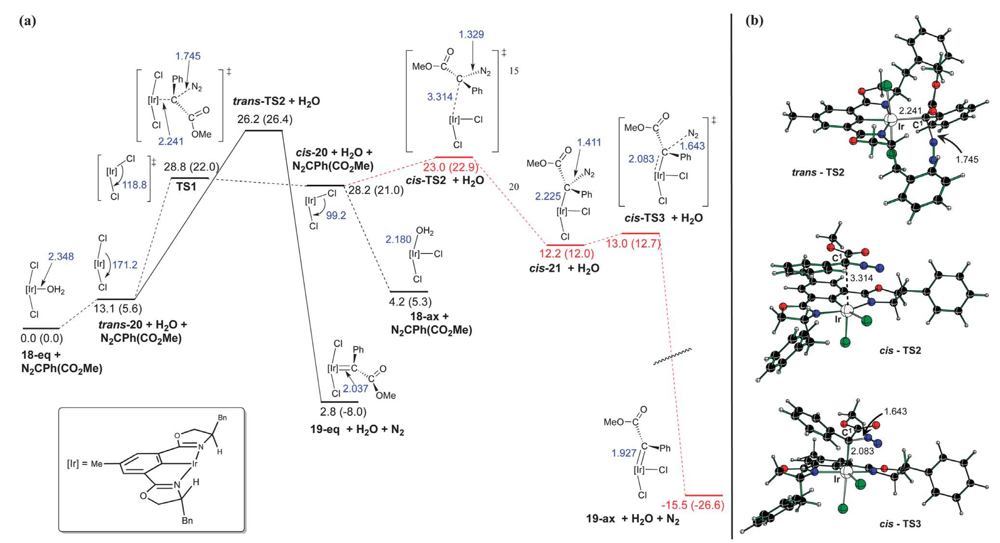
04/2013
Iridium(III)-bis(oxazolinyl)phenyl catalysts for enantioselective C–H functionalization
RESEARCH
-
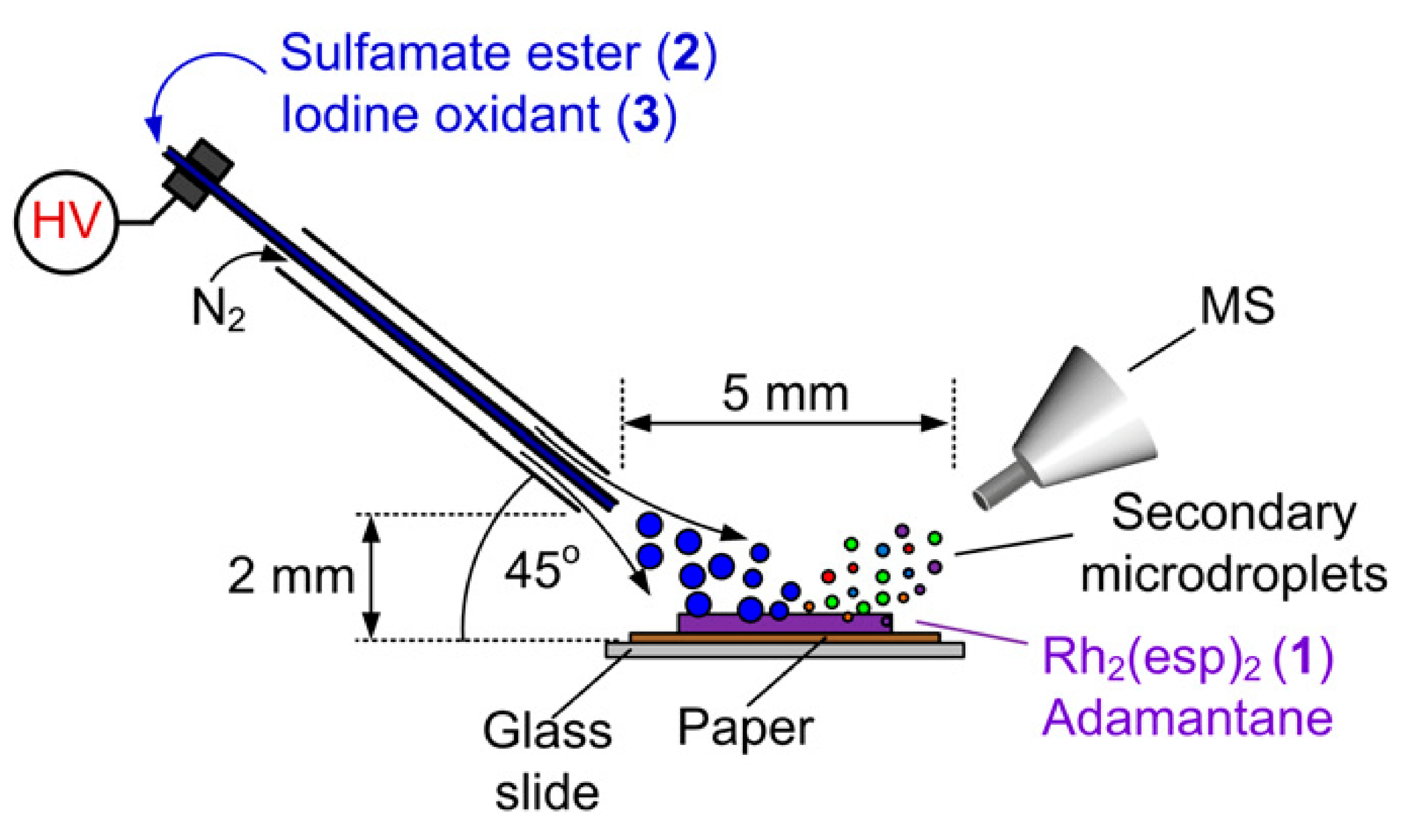
09/2012
Capturing fleeting intermediates in a catalytic C–H amination reaction cycle
RESEARCH
-
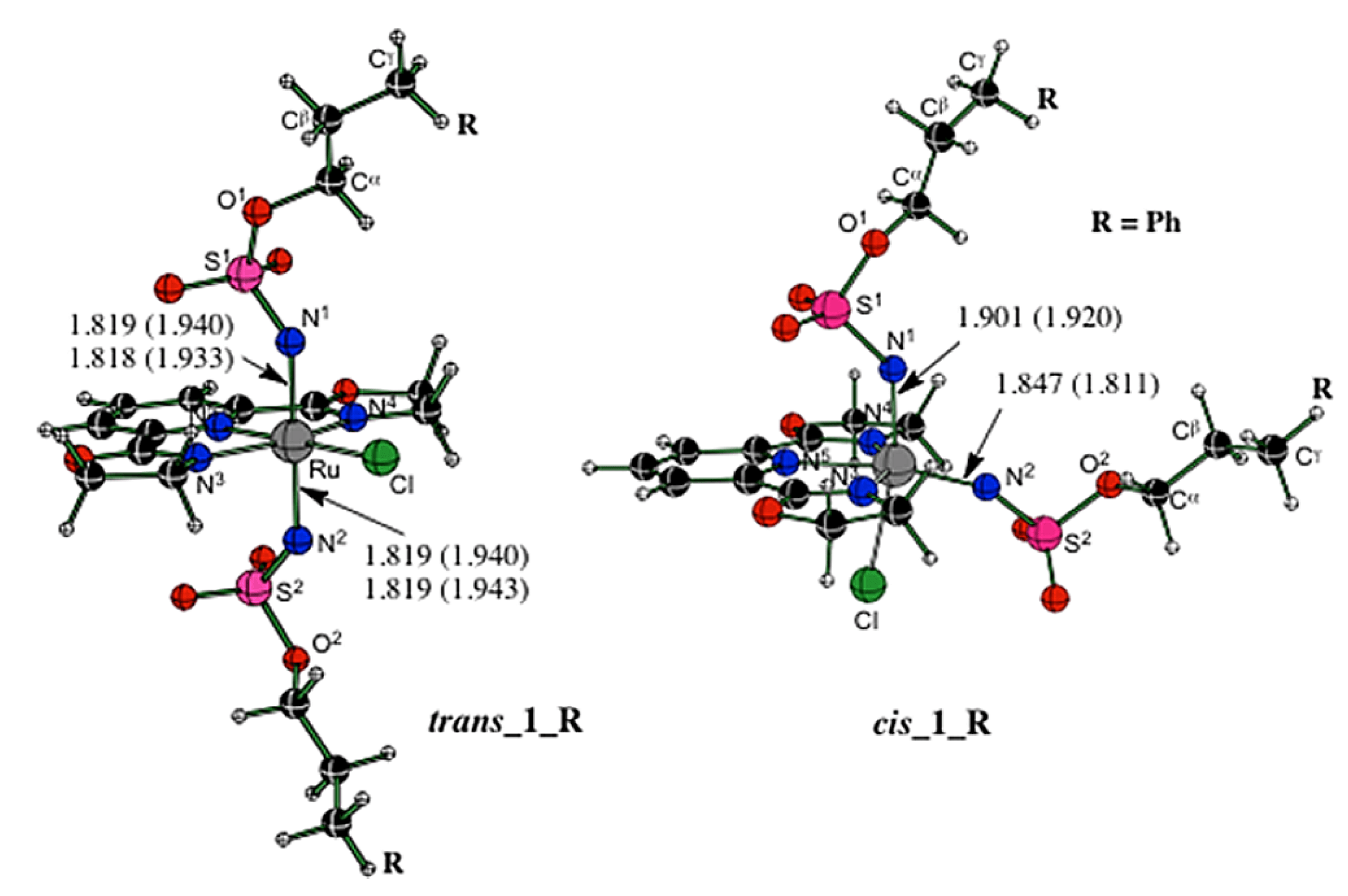
06/2012
Insight into Mechanistic Features of Ruthenium(II)-Pybox Catalyzed C-H Amination
RESEARCH
-
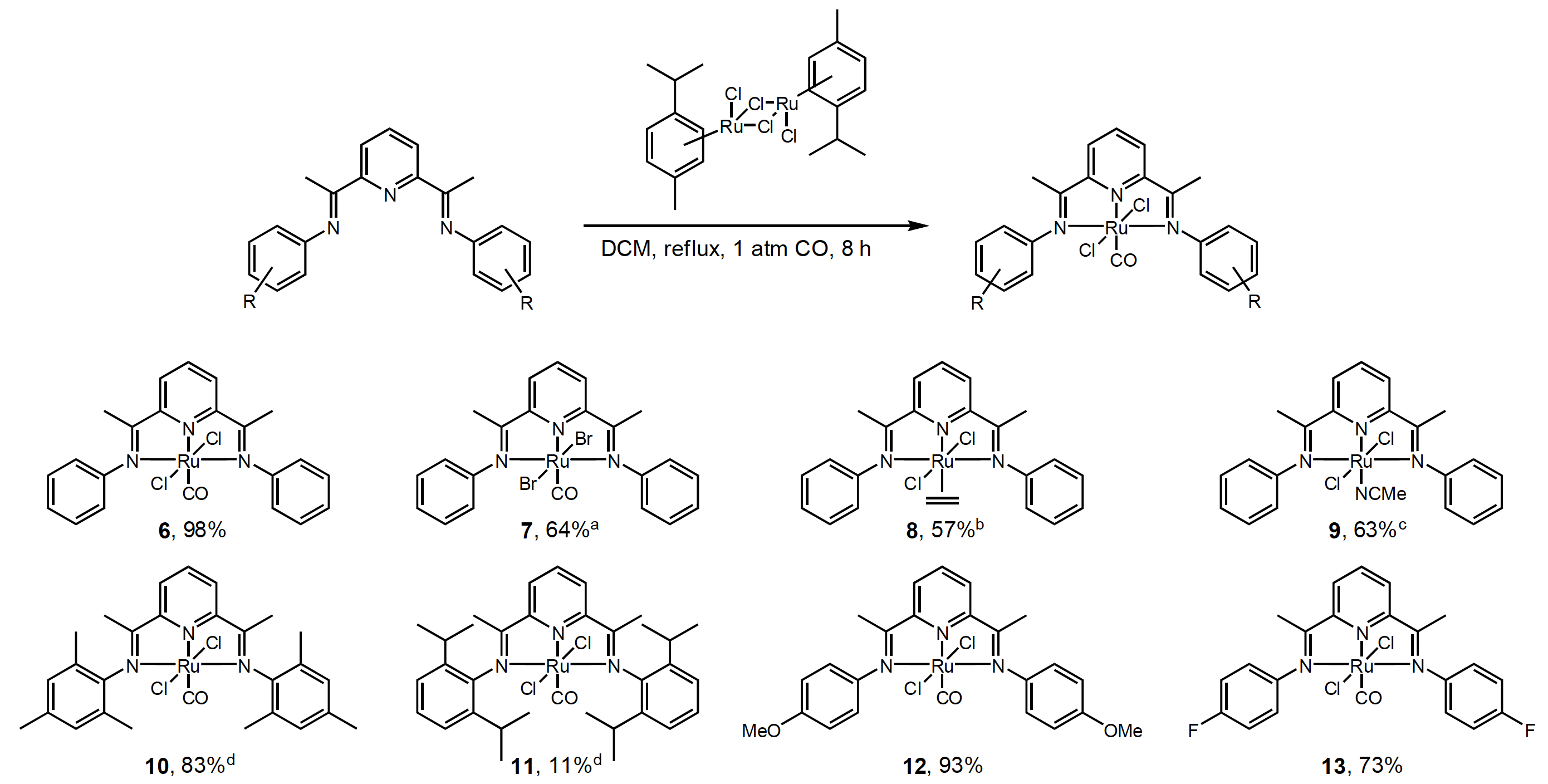
11/2011
Synthesis of Ruthenium(II)-2,6-Bisiminopyridyl Complexes for C-H Amination of Sulfamate Esters
RESEARCH
-
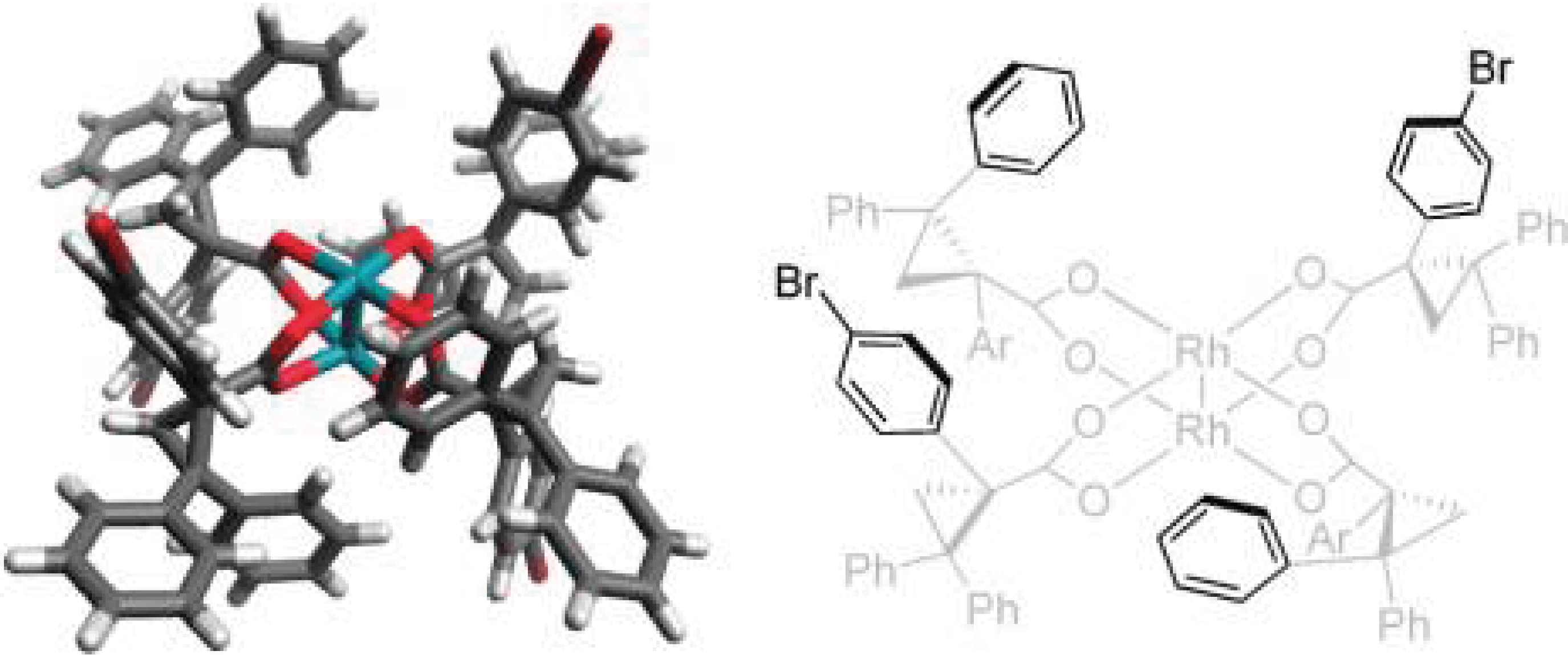
11/2011
D2-Symmetric Dirhodium Catalyst Derived from a 1,2,2-triarylcyclopropane carboxylate Ligand: Design, Synthesis and Application
RESEARCH
-
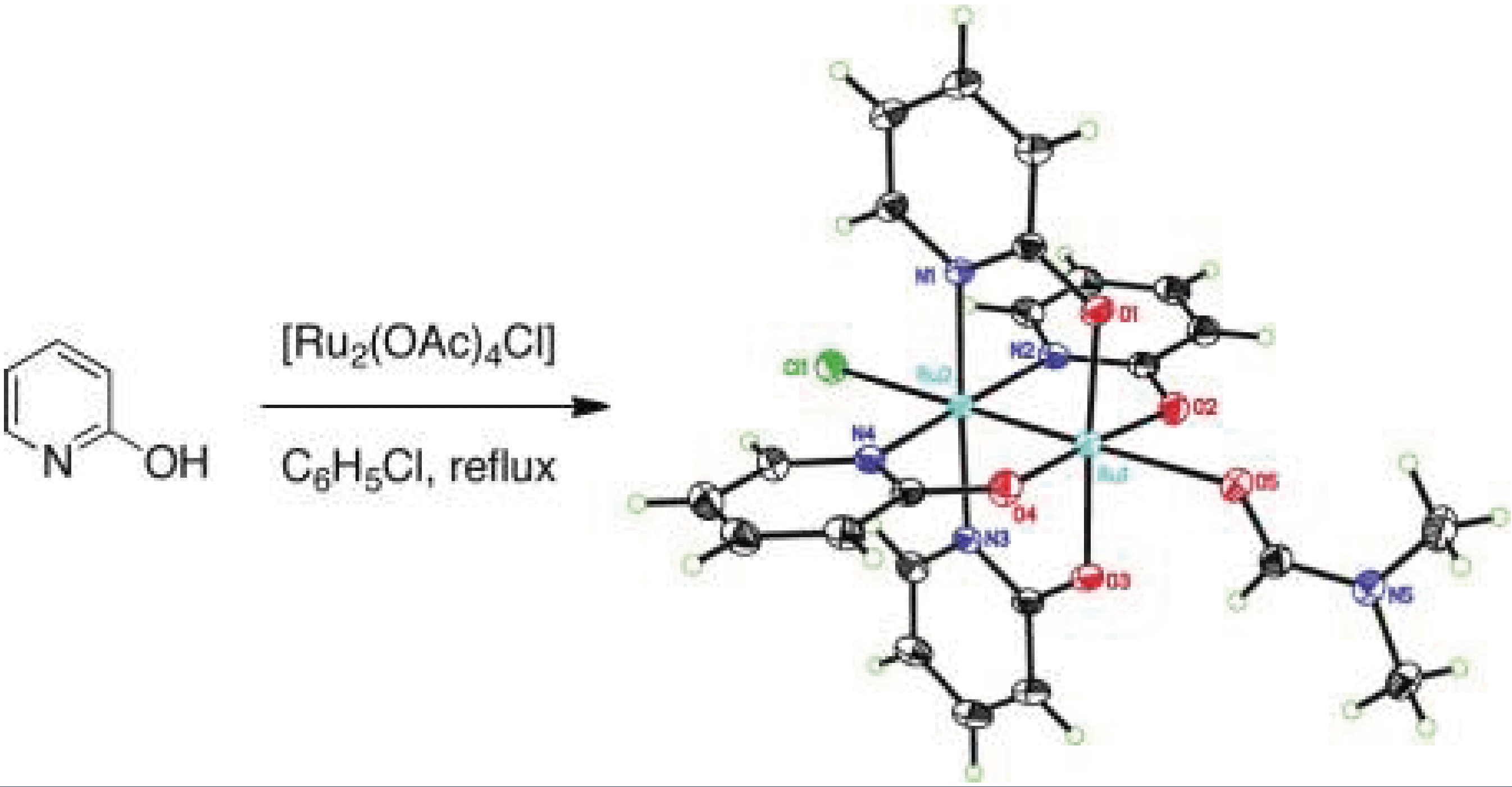
10/2011
A Diruthenium Catalyst for Selective, Intramolecular Allylic C-H Amination
RESEARCH
-
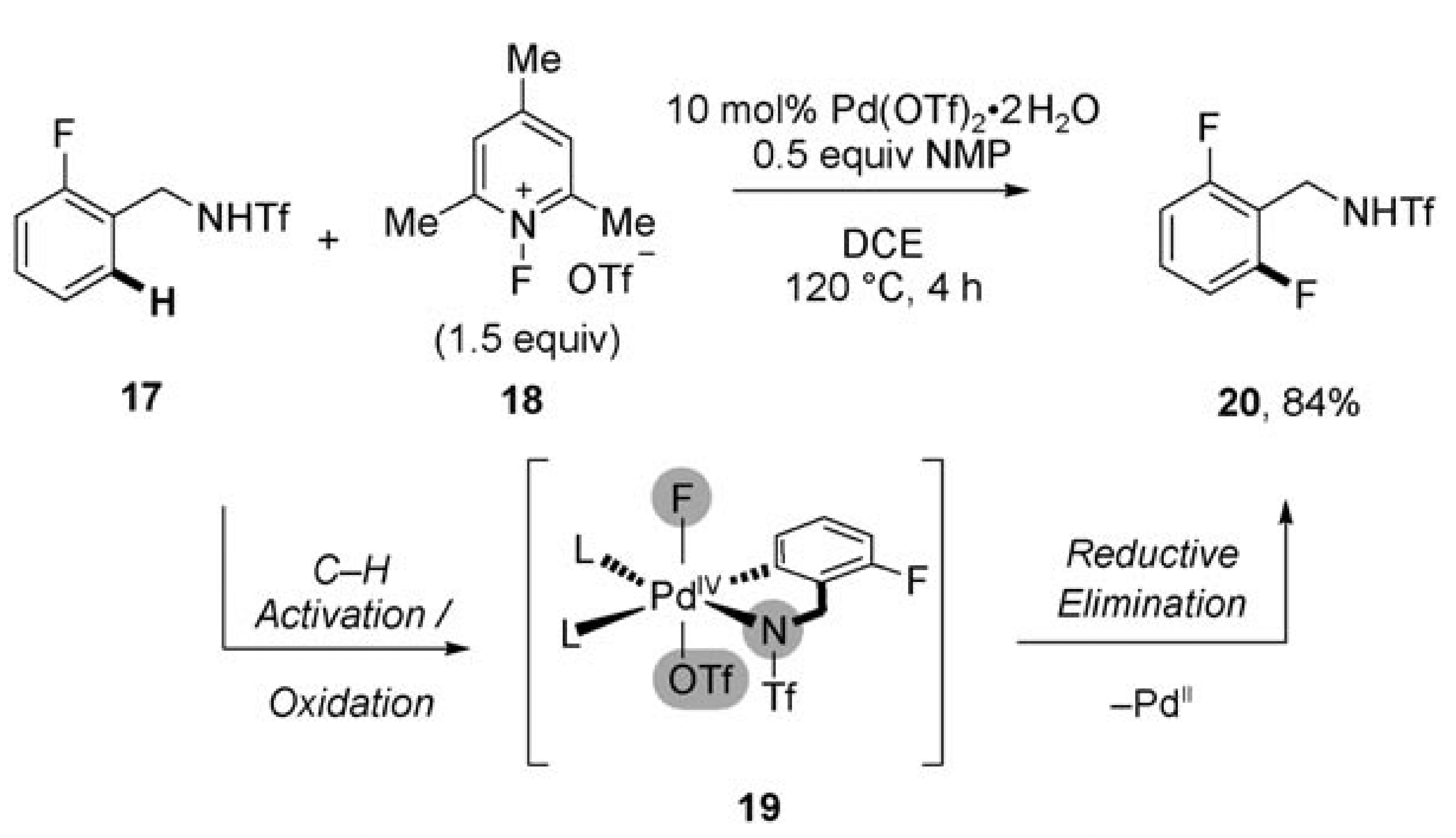
01/2011
Bystanding F+ Oxidants Enable Selective Reductive Elimination from High-Valent Metal Centers in Catalysis
RESEARCH
-
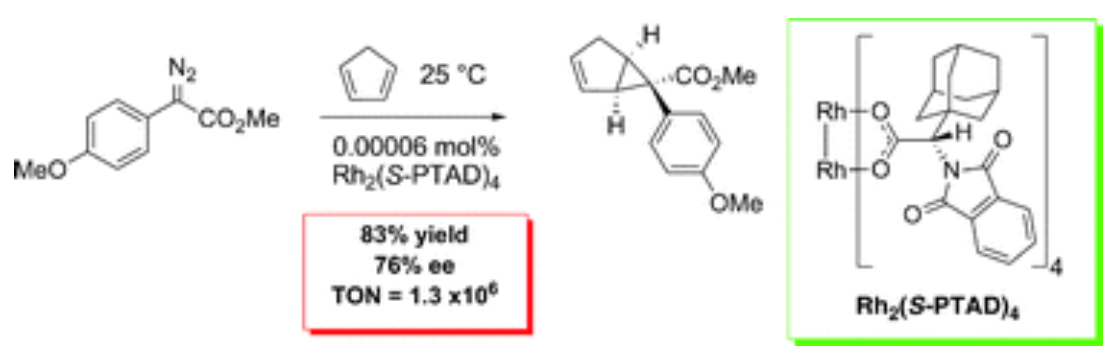
01/2010
Solvent-free catalytic enantioselective C–C bond forming reactions with very high catalyst turnover numbers
RESEARCH