Intermolecular sp3 C-H Amination of Complex Molecules
Nicholas Derek Chiappini, James B. C. Mack, Justin Du Bois
Angew. Chem. Int. Ed.,
2018, 57, 18, 4956-4959; DOI:10.1002/anie.201713225
02/2018
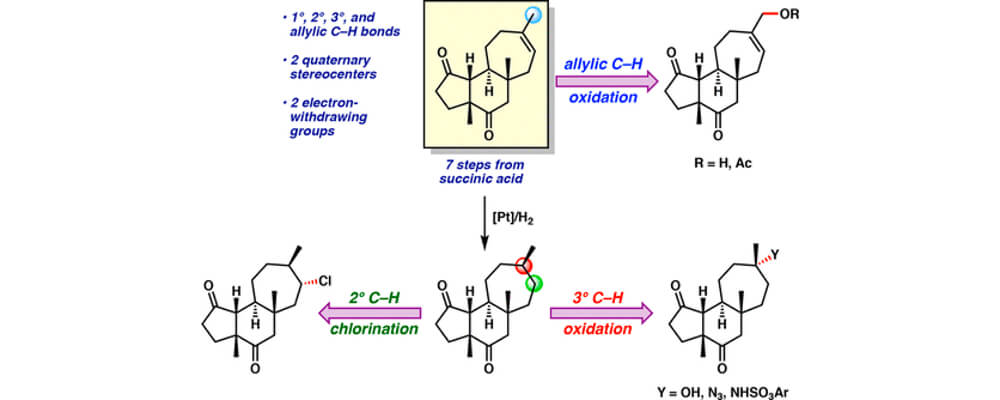
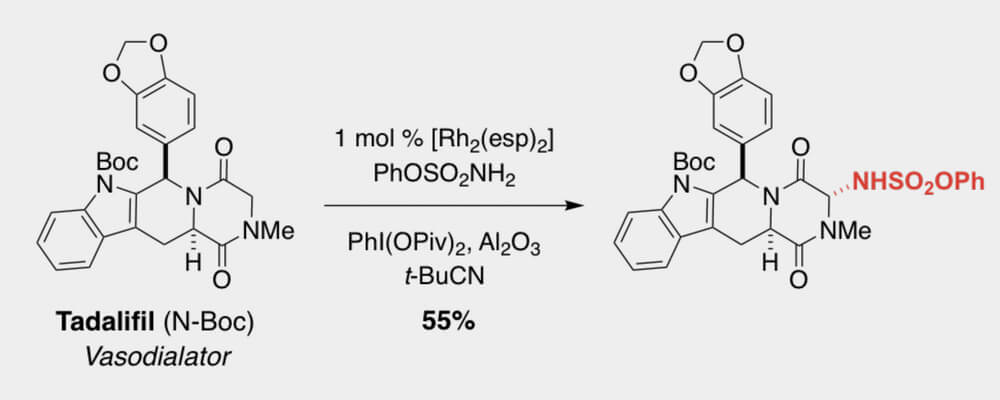
In this work, the Du Bois group discloses a new set of intermolecular sp3 C–H amination conditions utilizing pivalonitrile as solvent with greatly improved performance on a wide variety of complex molecule substrates, including numerous natural product and API derivatives.
The reaction is insensitive to air and moisture and does not require drying of solvent for optimal performance. In addition to tolerating common functionality and valuable synthetic handles such as pinacol boronate esters, heteroaryl bromides, electron rich arenes, oxadiazoles and some unprotected secondary alcohols, the reaction generally provides excellent mass balance, allowing for intact recovery of high-value substrates which do not proceed to completion. Product sulfamates are easily unmasked to the corresponding primary amines by heating with pyridine in wet acetonitrile while also tolerating other hydrolytically sensitive functionality.
Though the exact mechanism by which pivalonitrile is enhancing performance remains elusive, mechanistic investigations suggest that catalyst lifetime is significantly prolonged in pivalonitrile compared to other solvents tested. Additionally, solvent oxidation was found to have a marked role on catalyst performance as use of deuterated acetonitrile nearly doubled turnover seen in standard acetonitrile. Current efforts focus on further understanding mechanisms of catalyst arrest with the express goal of improving catalyst turnover.
Author: Nick Chiappini