Cyanthiwigin Natural Product Core as a Complex Molecular Scaffold for Comparative Late-Stage C–H Functionalization Studies
Kelly E. Kim, Ashley M. Adams, Nicholas D. Chiappini, J. Du Bois, and Brian M. Stoltz
J. Org. Chem.,
2018, 83, (6), 3023; DOI:10.1021/acs.joc.7b03291

01/2018
Obtained from natural sources such as plant leaves, marine animals, or jungle-dwelling creatures, natural products have served medicinal purposes in human civilization for centuries. While our ancient ancestors knew which plant leaves to collect for various ailments, today’s health professionals know which chemicals in the plant leaves are responsible for their healing effects, thanks to advances in science and technology. Armed with knowledge of the molecular structures of these natural medicines, scientists can study the relationship between molecular structure and therapeutic activity. This allows them to create potent pharmaceuticals based on natural products with therapeutic properties. Many natural products possess complicated molecular structures that require finesse and creative planning to construct in the laboratory. For this reason, chemists aiming to synthesize these compounds are excited to discover new ways to forge important chemical bonds as efficiently and effectively as possible.
One such method that has attracted a lot of interest is C–H bond functionalization. Traditionally, bonds between carbon and hydrogen are considered inert due to their relative strength and stability. However, the past few decades have seen an explosion of growth in the development of chemical reactions that break this rule. By installing useful molecular handles directly onto C–H bonds, chemists can avoid using intermediate functional groups common in traditional syntheses. This allows for shorter synthetic routes to important therapeutic compounds and reduced waste generation. However, a major challenge in employing C–H bond functionalization in the preparation of pharmaceuticals lies in selectively functionalizing one C–H bond over another. Many natural products and molecules with comparable structural complexity possess dozens of C–H bonds in their molecular architectures. To address this, chemists have developed reactions that target certain types of C–H bonds over others for functionalization.
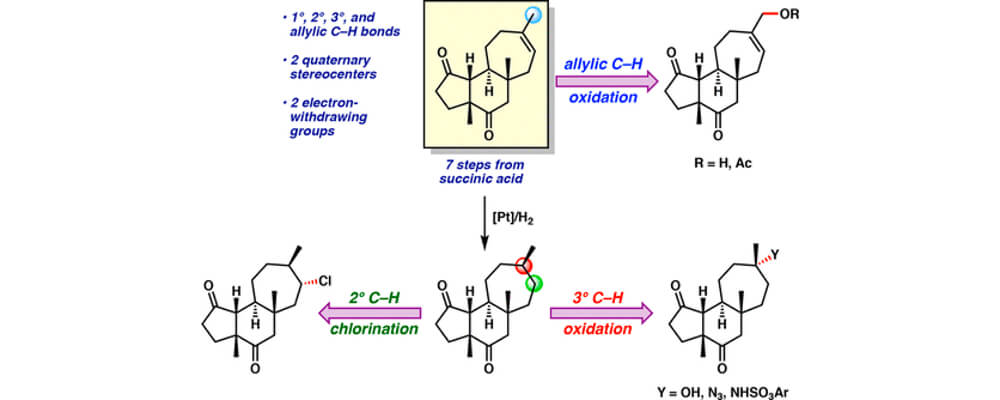
Years of research effort have produced a wealth of methods for C–H functionalization available for use in synthetic planning. However, there are few accounts comparing the efficacies of diverse methods on a single complex molecule. The study described in this article accomplishes this by applying various well-established methods for C–H functionalization to the tricyclic carbon framework of the cyanthiwigin natural product family, a class of compounds found in sea sponges. This side-by-side comparison illustrates which methods work most effectively on the natural product scaffold and which C–H bonds within the molecule react most readily under those conditions. The findings from this collaborative effort between the Stoltz group at Caltech and the Du Bois group at Stanford offer guidance to others aiming to employ C–H functionalization strategies in chemical synthesis. Ultimately, greater applicability of C–H functionalization in complex molecule synthesis would lead to increased efficiency and broader capabilities in the preparation of important therapeutics.
The article was highlighted as an ACS Editor's Choice, with the article remaining as open access.
Author: Kelly Kim
Related Content
-
02/2018
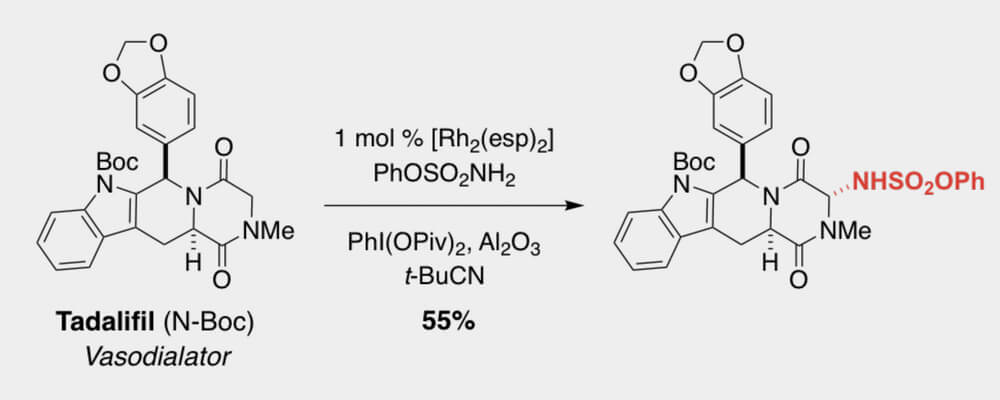
Intermolecular sp3 C-H Amination of Complex Molecules
RESEARCH
-

10/2017
OPEN: Selective Oxidation of C–H Bonds with Hyper-Electrophiles
EDUCATION
-
10/2016
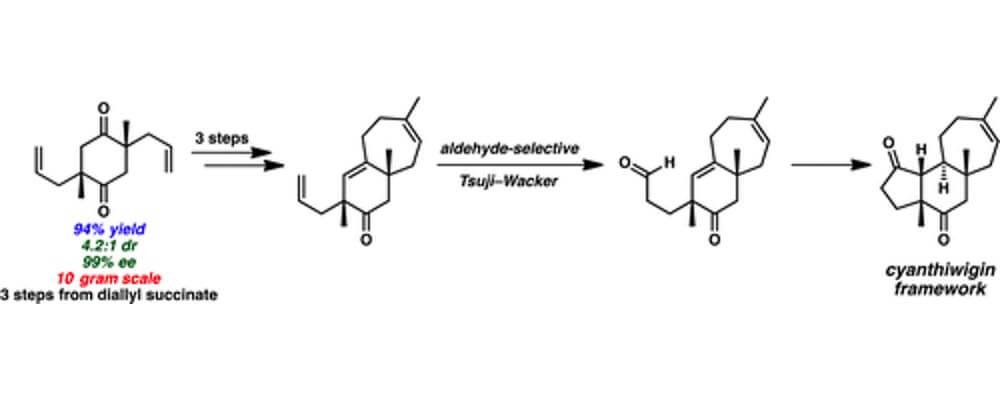
A Second-Generation Synthesis of the Cyanthiwigin Natural Product Core
RESEARCH
-
06/2013
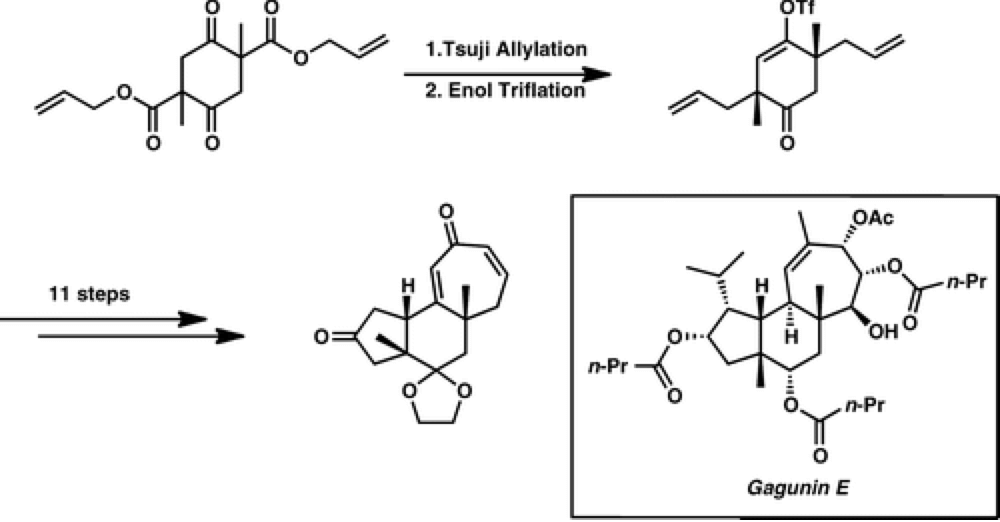
Enantioselective Synthesis of the 5–6–7 Carbocyclic Core of the Gagunin Diterpenoids
RESEARCH