Iridium(III)-bis(imidazolinyl)phenyl catalysts for enantioselective C–H functionalization with ethyl diazoacetate
N. Mace Weldy, A. G. Schafer, C. P. Owens, C. J. Herting, A. Varela-Alvarez, S. Chen, Z. Niemeyer, D. G. Musaev, M. S. Sigman, H. M. L. Davies and S. B. Blakey
Chemical Science
2016, 7, 3142-3146; 10.1039/C6SC00190D
02/2016
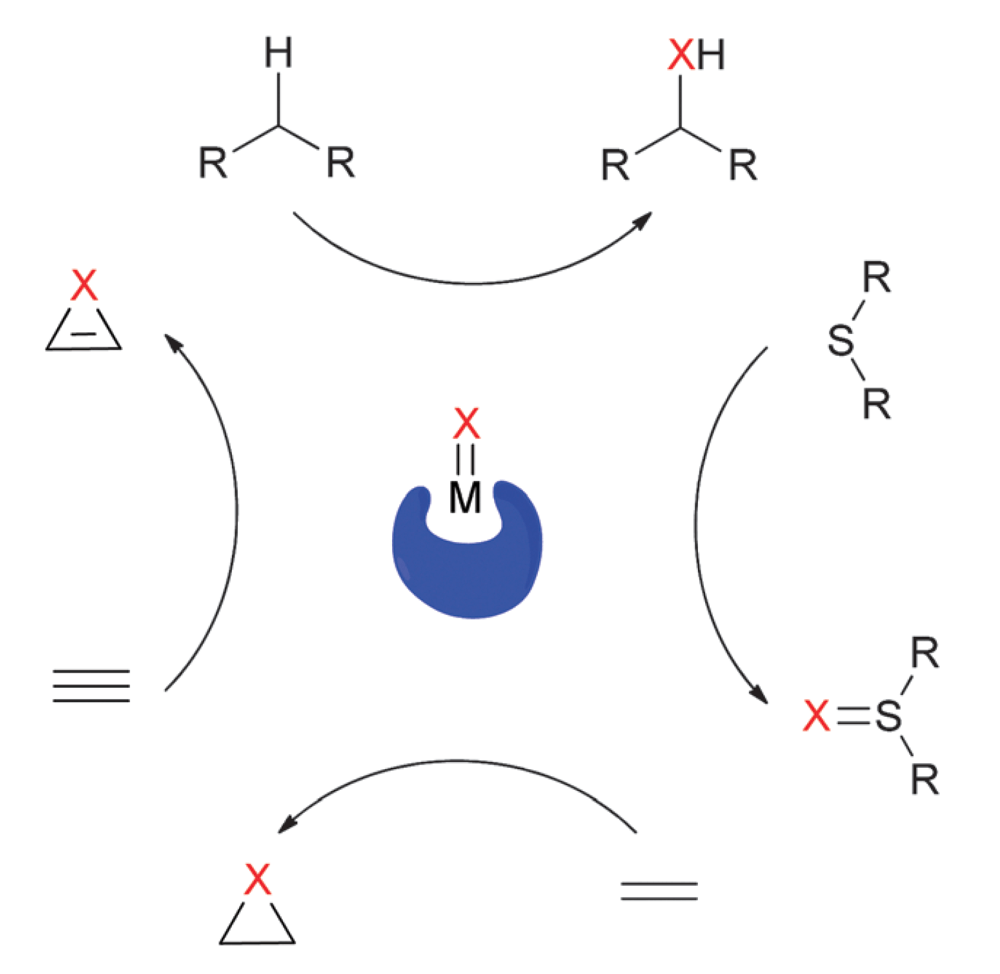
Insertion of donor/acceptor carbenes into C–H bonds has become a powerful transformation for synthetic chemistry. The donor group tempers the electrophilicity of the metallocarbene and imparts exquisite regio- and enantiocontrol in these reactions.
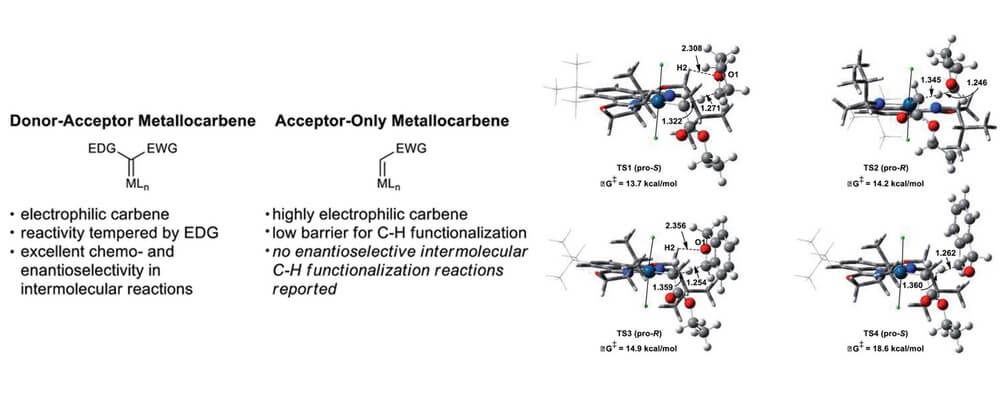
In contrast, there are no reports of enantioselective intermolecular C–H functionalization reactions employing acceptor-only carbene precursors, despite the fact that these reagents are arguably more readily available.
This collaborative study brings together the Blakey, Sigman, Davies and Musaev group to leverage a broad skill set within the CCHF to design a catalyst system capable of addressing this challenge. Two key factors were identified for this system; these metallocarbenes are highly electrophilic and reactive and the acceptor-only carbene itself is not prochiral, and thus in order to achieve an enantioselective reaction, the catalyst must provide an environment capable of differentiating the incoming orientations of the substrate.
This work brings experimental, theoretical and linear regression techniques to pull apart the factors that impact the selectivity in this system, and furnishes a catalyst system capable of highly regio- and stereoselective carbene C–H insertion. This is a significant expansion in the field of carbene C–H insertion and will inform the design of new catalyst systems.