Concise Total Synthesis of (+)-Luteoalbusins A and B
Timothy C. Adams, Joshua N. Payette, Jaime H. Cheah, and Mohammad Movassaghi
Organic Letters,
2015, 17 (17), pp 4268–4271; 10.1021/acs.orglett.5b02059
08/2015
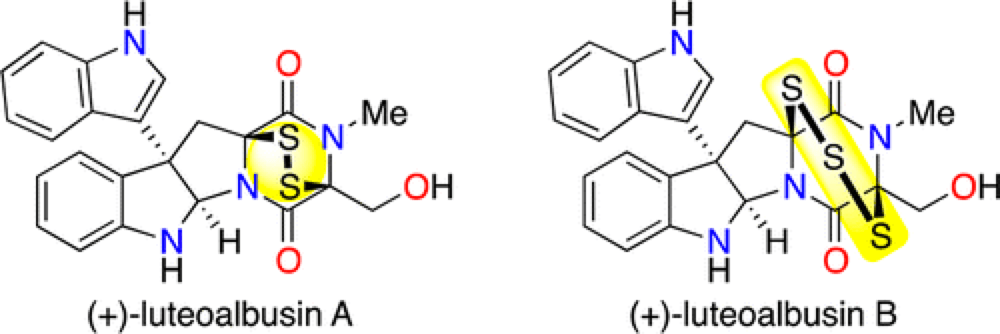
The Epipolythiodiketopiperizine (ETP) alkaloids represent a structurally complex class of secondary fungal metabolites that have demonstrated remarkable activity against a number of human cancer cell lines. While members of this class share a number of core structural features, they also include a wide variety of structural diversity, both in terms of the side-chain structure and the level of oxidation of the core scaffold. Thus any strategy toward this class of compounds must be modular and versatile in nature to be adaptable to the variety of functionality present in this family.
The Movassaghi group have an established history working in this field and they have brought this experience to the first total synthesis and assessment of the Natural products (+)-luteoalbusin A and B.
The versatile strategy that they have developed toward these molecules employs a late-stage permanganate-mediated stereoretentive C–H hydroxylation, installing the functionality required to assemble the polysulfide bridge.
This strategy highlights the versatility offered by a late-stage C–H functionalization strategy, allowing a modular and adaptable approach to complex structures.