Cu(II)-Mediated C(sp2)–H Hydroxylation
Shang-Zheng Sun, Ming Shang, Hong-Li Wang, Hai-Xia Lin, Hui-Xiong Dai, and Jin-Quan Yu
J. Org. Chem.,
2015, 80 (17), pp 8843–8848; 10.1021/acs.joc.5b01351
08/2015
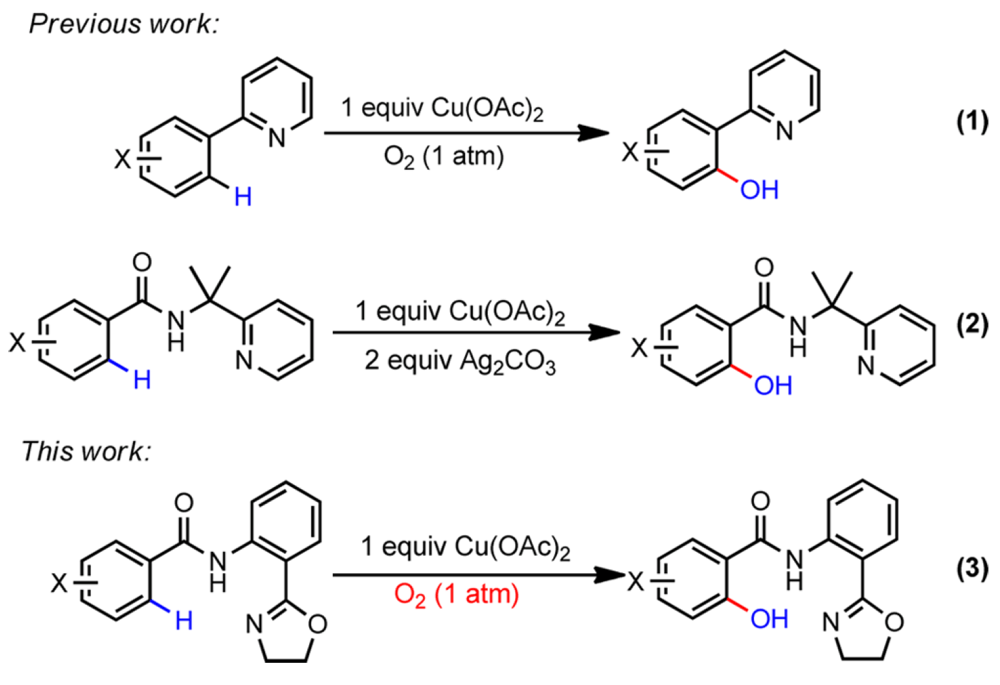
The ubiquitous nature of phenols, found throughout pharmaceutical agents, natural products and molecular materials and their versatility in organic synthesis and catalysis has made methods for their synthesis an area of significant interest. This report from the Yu and Dai groups details their collaborative work on the development of a copper-catalyzed C(sp2)–H hydroxylation chemistry that employs a weakly-coordinating and removable directing group across a broad scope of substrates.
Previous work in this field required the use of either palladium- or ruthenium-catalyzed systems, even then the reactions suffered from poor conversions, low levels of selectivity, harsh reaction conditions and the use of strongly-coordinating directing groups that could not subsequently be removed.
During their studies exploring the copper-catalyzed alkynatiion of C(sp2)–H bonds the Yu and Dai groups observed significant levels of the then undesired hydroxylated byproduct. Recognizing this as a potentially useful lead this work describes how they have optimized the conditions of this reaction to provide a selective and mild high yielding C(sp2)–H hydroxylation technology, using a weakly coordinating directing group that can be readily removed, under conditions that use molecular oxygen as the oxidant.
This provide a significant methodology for those seeking to use C–H functionalization to construct complex molecules that employs cheap and abundant copper as the catalyst.