Mechanistic analysis of an asymmetric palladium-catalyzed conjugate addition of arylboronic acids to ß-substituted cyclic enones
Cornelia Leonie Boeser, Jeffrey C. Holder, Buck L. H. Taylor, K Houk, Brian M. Stoltz and Richard N. Zare
Chemical Science,
2015, 6, 1917; 10.1039/C4SC03337J
12/2014
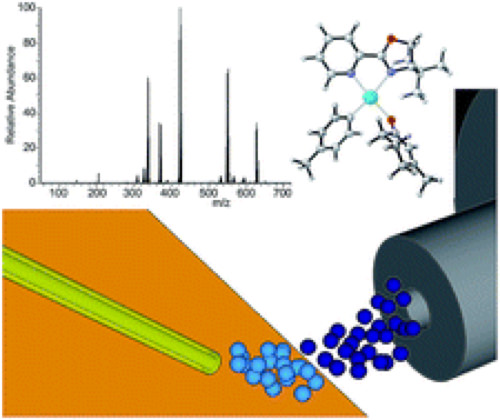
The Stoltz, Zare and Houk group have brought a collaborative approach to deduce the mechanism of an asymmetric palladium-catalyzed conjugate addition of arylboronic acids to enone substrates.
DESI-MS identified some of the key intermediates of the catalytic cycle and helped to identify some of the reactivity trends observed in the substrates. A combination of DFT calculations and experimental observations helped confirm these conclusions.