An Enantiospecific Synthesis of Jiadifenolide
David A. Siler, Jeffrey D. Mighion and Prof. Erik J. Sorensen
Angew. Chem. Int. Ed.,
2014, 53, (21), 5332-5335; 10.1002/anie.201402335
04/2014
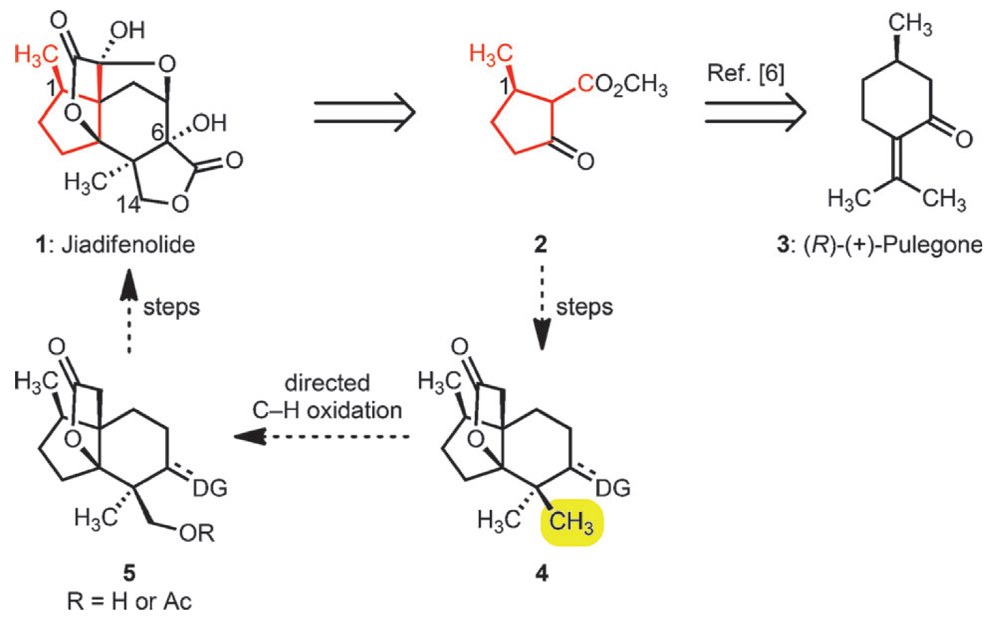
Jiadifenolide is a chemical constitutent of the flowering plant Illicium jiadifengpi boasting an unusual, highly oxidized pentacyclic cage-like structure. This class of natural products, and in particular Jiadifenolide, have been found to significantly potentiate neurite outgrowth in rats, essentially helping the regrowth of brain cells.
The Sorensen group have completed a concise and elegant synthesis of this molecule, using a fusion of traditional and cutting-edge chemistry to access this complex scaffold. Starting from the readily available monoterpene (+)-pulegone the second ring of the structure was introduced using a Robinson Annulation, one of the classic ring-forming reactions of total synthesis.
The key reaction was the selective oxidation of methyl group. By using a C–H oxidation strategy this group could be carried through the synthesis as one of germinal methyl pair, desymmetrizing the synthesis at a late stage. This was accomplished using chemistry developed by the Stanford group, albeit in low diastereoselectivity. The reaction proceeded in high conversion and the products could be resolved using chromatography. This supplied access to a late stage intermediate that was successfully taken on to the natural product.
This approach demonstrates a highly concise entry into this class of natural products, using C–H Functionalization to not only facilitie the synthesis by carrying through the gem-dimethyl groups, but also introduce required functionality at a late stage.