Dichotomous Hydrogen Atom Transfer vs Proton-Coupled Electron Transfer During Activation of X–H Bonds (X = C, N, O) by Nonheme Iron–Oxo Complexes of Variable Basicity
Dandamudi Usharani, David C. Lacy, A. S. Borovik, and Sason Shaik
J. Am. Chem. Soc.,
2013, 135, 17090-17104; 10.1021/ja408073m
10/2013
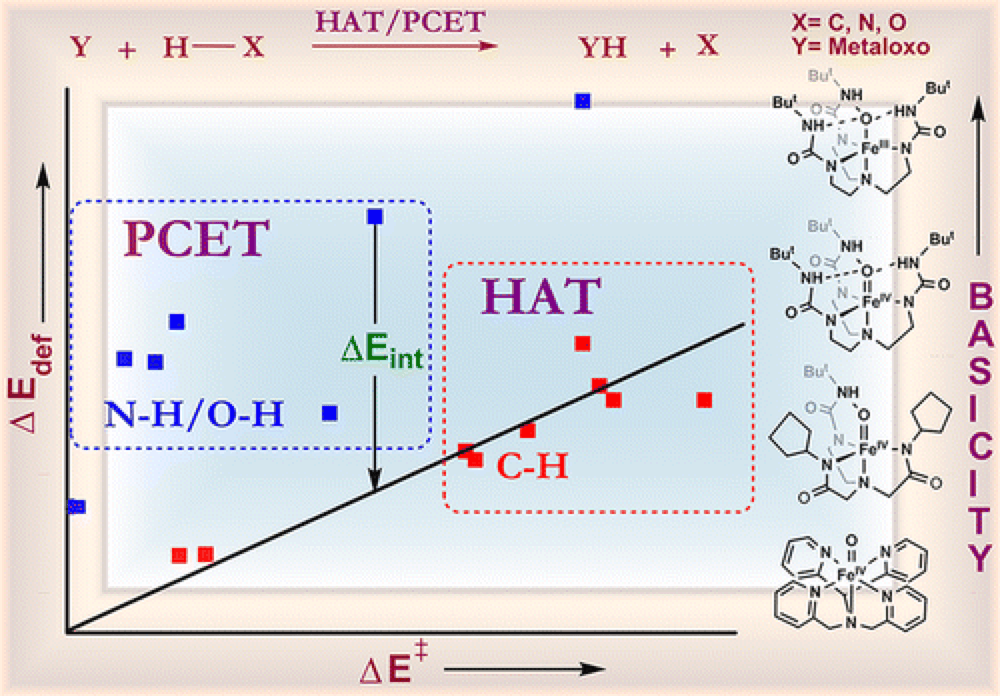
The Borovik group, in collaboration with the Shaik group, report their findings on the impact of the basicity of a given iron-complex on the reaction pathway in C–H, N–H and O–H bond activation systems.
Through a combination of experimental observations and theoretical findings a comparative picture is constructed of a mechanistic spectrum ranging from concerted hydrogen atom transfer (HAT) and proton-coupled electron-transfer (PCET) mechanisms, through asynchronous proton-transfer (PT)/electron transfer (ET), all the way to PT.
Using a valence bond model the observed results are linked directly to the basicity/acidity of the reagents, but also highlight significant differences in the fundamental pathways of C–H versus N–H and O–H bond functionalization.