On the Mechanism of Permanganate Promoted Dihydroxylation of Complex Diketopiper-azines: Critical Roles of Counter-Cation and Ion-Pairing
Brandon E. Haines, Brandon M. Nelson, Jessica M. Grandner, Justin Kim,
Kendall N. Houk, Mohammad Movassaghi, Djamaladdin G. Musaev
J. Am. Chem. Soc.,
2018, 140 (41), 13375–13386; DOI:10.1021/jacs.8b08371
10/2018
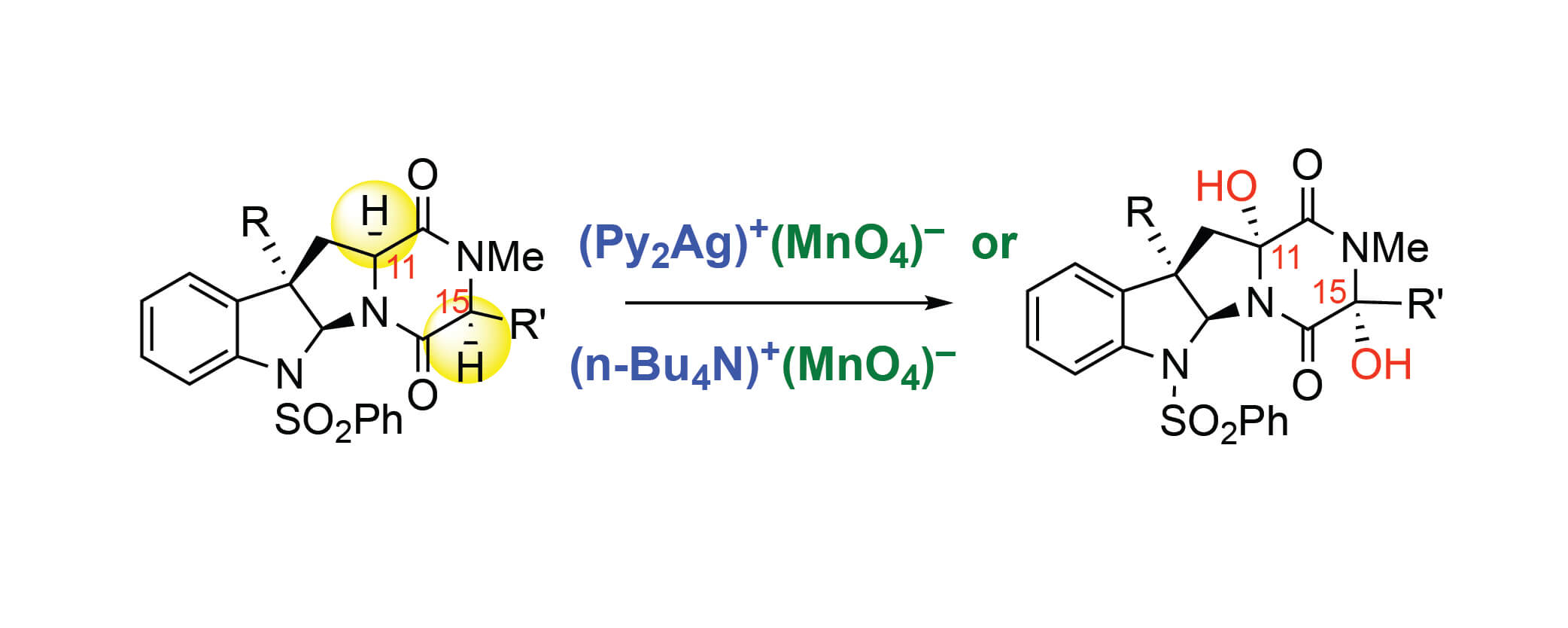
The mechanism of permanganate-mediated dual C–H oxidation of complex diketopiperazines has been examined with density functional theory computations. The products of these oxidations are enabling intermediates in the synthesis of structurally diverse ETP natural products. We evaluated, for the first time, the impact of ion-pairing and aggregation states of the permanganate ion and counter-cations, such as bis(pyridine)-silver(I) (Ag+) and tetra-n-butyl ammonium (TBA+), on the C–H oxidation mecha-nism. We confirmed the previously proposed the C–H abstraction followed O-rebound mechanism lead-ing to a permanganate ester intermediate, but also found that there can be competing OH-rebound pathway leading to the alcohol product. It is shown that the C–H abstraction occurs through an open shell singlet species followed by O-rebound and a competing OH-rebound pathway. The second C–H oxidation proceeds with a second equivalent of oxidant with lower free energy barriers than the first C–H oxidation due to directing effects and the generation of a more reactive oxidant species after the first C–H oxidation.
The success and efficiency of the second CH oxidation is found to be critically dependent on the pres-ence of an ion-paired oxidant. We used the developed mechanistic knowledge to rationalize an experi-mentally observed oxidation pattern for C3-indole substituted diketopiperazine under optimal oxidation conditions: namely, the formation of diol as a single diastereomer and lack of the ketone products. We proposed two factors that may impede the ketone formation: (i) the conformational flexibility of the diketopiperazine ring, and (ii) hindrance of this site, making it less accessible to the ion-paired oxidant species.