Intermolecular Allylic C−H Etherification of Internal Olefins
Taylor A. F. Nelson and Simon B. Blakey
Angew. Chem. Int. Ed.,
2018, 130, 45, 15127-15131; DOI:10.1002/ange.201809863
09/2018
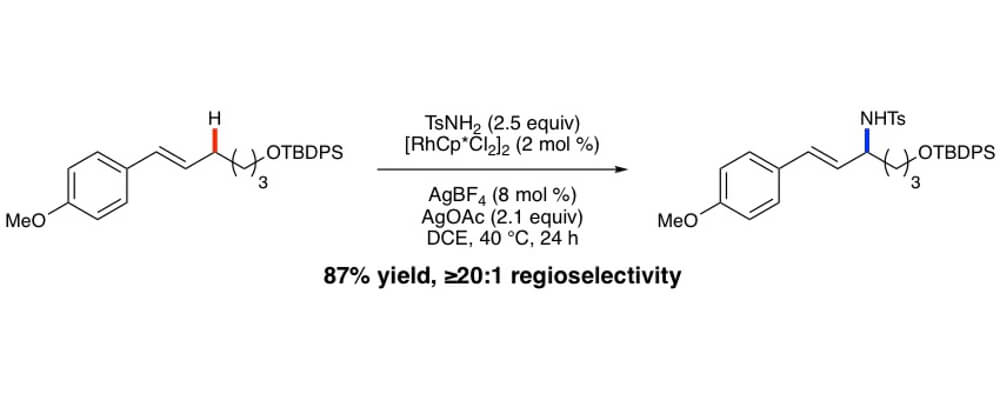
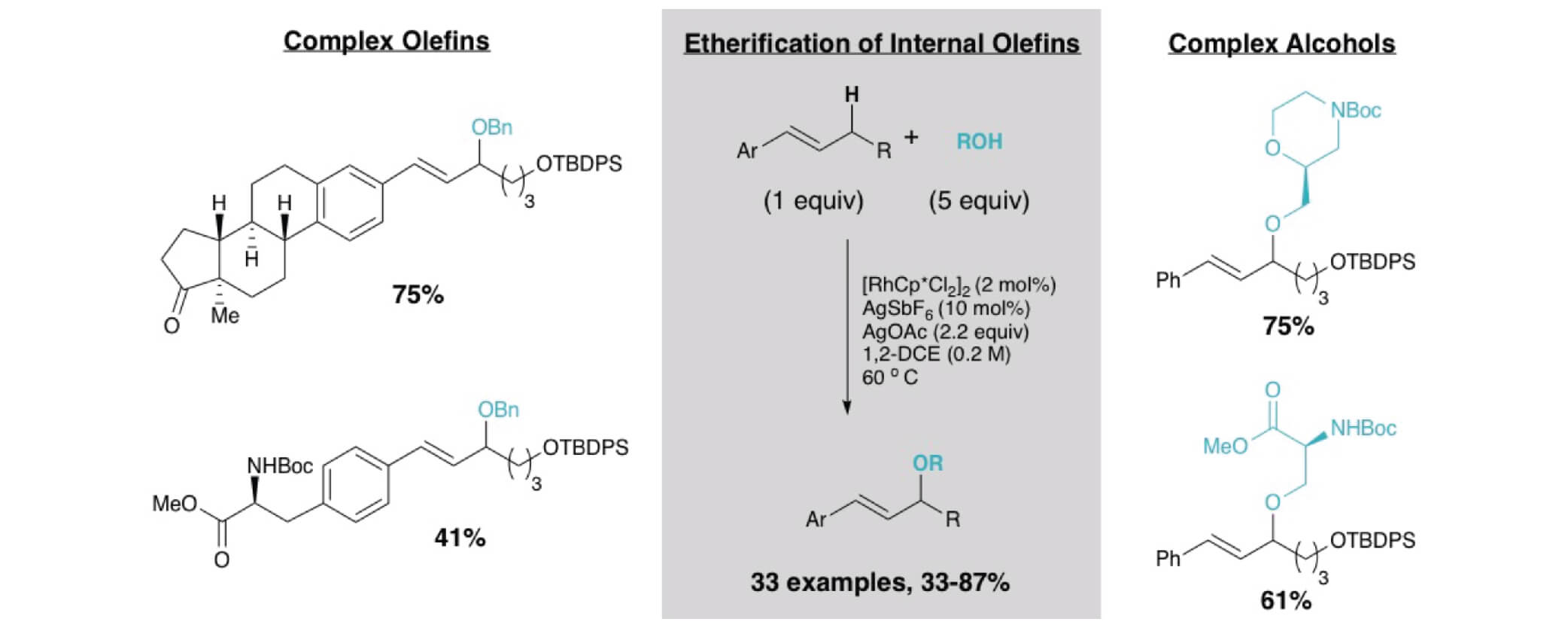
Herein we report on the development of an oxidative allylic C−H etherification reaction, utilizing internal olefins and alcohols as simple precursors. Late stage C–H functionalization of complex olefins would allow for the synthesis of diverse molecular libraries quickly.
Key advances include the use of RhCp* complexes to promote the allylic C−H functionalization of internal olefins and the compatibility of the oxidative conditions with oxidatively sensitive alcohols, enabling the direct etherification reaction. Many pharmacophores including oxetanes, azetidines, sugars, serine, and morpholine derivatives were compatible as the alcohol coupling partner in this system showcasing the potential. More biologically relevant olefin coupling partners incorporate phenylalanine, estrone, and vitamin D.
Preliminary mechanistic studies include kinetic isotope studies demonstrating a primary KIE consistent with C–H cleavage as the rate determining step. A secondary KIE indicating the rehybridization from sp2 to sp3 was also observed and is consistent with a π-allyl intermediate. Deuterium exchange studies confirmed that C–H cleavage was irreversible. This transformation establishes allylic C–H functionalization as a viable disconnection in synthetic strategies.