Harnessing the β-Silicon Effect for Regioselective and Stereoselective Rhodium(II)-Catalyzed C–H Functionalization by Donor/Acceptor Carbenes Derived from 1-Sulfonyl-1,2,3-triazoles
Zachary J. Garlets and Huw M. L. Davies
Org. Lett.,
2018, 20 (8), pp 2168–2171; DOI: 10.1021/acs.orglett.8b00427
04/2018
Organosilanes are widely represented in a variety of chemical disciplines including agrochemistry, materials science, and pharmaceutical chemistry. Despite the prevalence of silanes in these diverse fields, most methods rely on polar reactions to construct silanes.
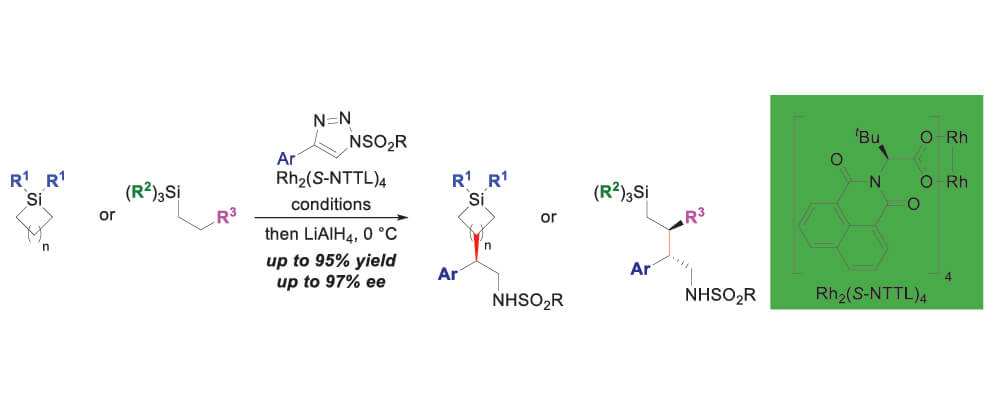
In this study, the ability of silicon to stabilize the build-up of positive charge at the β position was utilized to accomplish regioselective and stereoselective C–H insertion reactions by dirhodium tetracarboxylate carbenes derived from 1-sulfonyl-1,2,3-triazoles. While levels of regioselectivity are generally high for different catalysts, the use of Rh2(S-NTTL)4 was able to achieve the highest levels of enantioselectivity.
The reaction was effective for a variety of different triazoles, and silanes. Notably, the functionalization of triethylsilane was achieved at the primary position which is notoriously difficult because positive charge development is less stabilized. In addition, diastereoselectivity was modest in this reaction, and so further efforts are underway to design catalysts which improve the diastereoselectivity to more synthetically useful levels.