Ligand-enabled ortho-C–H olefination of phenylacetic amides with unactivated alkenes
Ming-Zhu Lu, Xing-Rong Chen, Hui Xu, Hui-Xiong Dai and Jin-Quan Yu
Chem. Sci.,
2018, 9, 1311-1316; DOI: 10.1039/C7SC04827K
01/2018
One of the most successful strategies for controlling the site of reaction in C–H functionalization is through the formation of a temporary and reversible complex between the substrate, the catalyst-bound reagent and a ligand. This assembly places the reactive molecule in close proximity to the desired C–H bond. Early studies that employed this strategy called the approach “directed C–H activation”, but as this field has developed and more sophisticated reactions systems have been designed, the term now used that better describes the strategy is “chelation-assisted”.
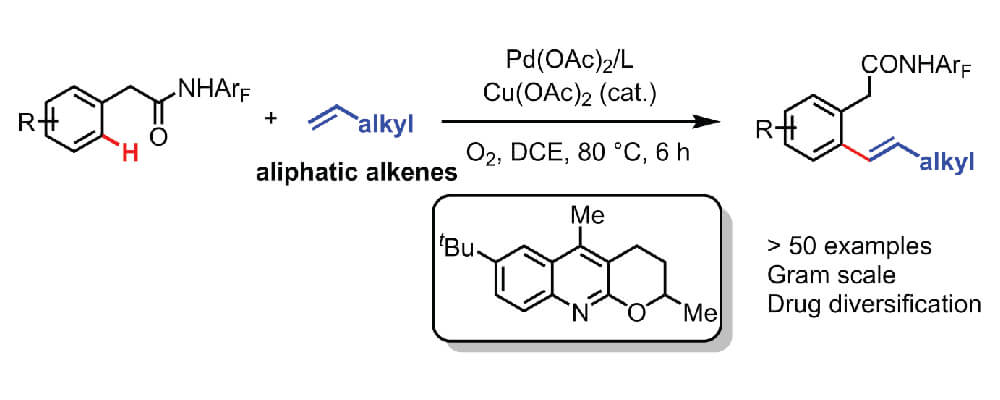
The Yu group is one of the pioneers in this field and this report describes a significant advance on a previously established reaction. Though chelation-assisted C–H olefination has been intensively investigated and employed in the past, Pd(II)-catalyzed C–H olefination reaction have largely been restricted to acrylates and styrenes. In this work the use of a quinoline-derived ligand and a weakly coordinating monodentate amide auxiliary enables the use of simple aliphatic alkenes as the reagents. Further enhancing the attractiveness of this reaction is the use of molecular oxygen as the terminal oxidant, with catalytic copper as the co-oxidant.
This new technique facilitates the instillation of alkenes bearing a wide range of functional groups. The utility of this approach is demonstrated through the late-stage diversification of drug molecules.