Understanding Regiodivergence in a Pd(II)-Mediated Site-Selective C–H Alkynylation
Kenji Usui, Brandon E. Haines, Djamaladdin G. Musaev, and Richmond Sarpong
ACS Catalysis,
2018, 8 (5), pp 4516–4527; DOI: 10.1021/acscatal.8b01116
04/2018
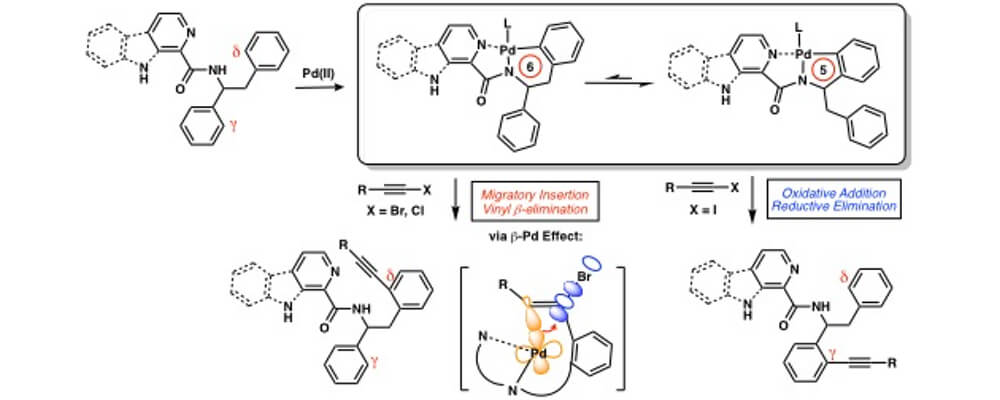
The Musaev and Sarpong groups report regiodivergence in a Pd(II)-mediated C(sp2)–H alkynylation of substrates with beta-carbolinamide and picolinamide as N,N-bidentate chelating groups based on the identity of the TIPS-alkyne-halide coupling partner: TIPS-alkyne-bromides give delta-C(sp2)–H alkynylation products while TIPS-alkyne-iodides give gamma-C(sp2)–H alkynylation products. Using an integrated experimental and computational approach, we examine the C–H alkynylation mechanism and determine the factors leading to the observed selectivity in great detail.
It was found that TIPS-alkyne-bromides react selectively with the six-membered palladacycle (from beta-palladation) through a migratory insertion pathway. We demonstrated that the gamma-selectivity observed with TIPS-alkyne-iodides arises from a switch in mechanism, from migratory insertion to Pd(II)/Pd(IV) oxidative addition. Lastly, the in-depth mechanistic study uncovered a beta-Pd effect, which is analogous to the well-established beta-Si effect, that promotes delta-C–H alkynylated product formation through an unusual vinyl beta-halo elimination.