Application of diazene-directed fragment assembly to the total synthesis and stereochemical assignment of (+)-desmethyl-meso-chimonanthine and related heterodimeric alkaloids
Stephen P. Lathrop, Mohammad Movassaghi
Chemical Science
2013, 5, 333-340; 10.1039/C3SC52451E
09/2013
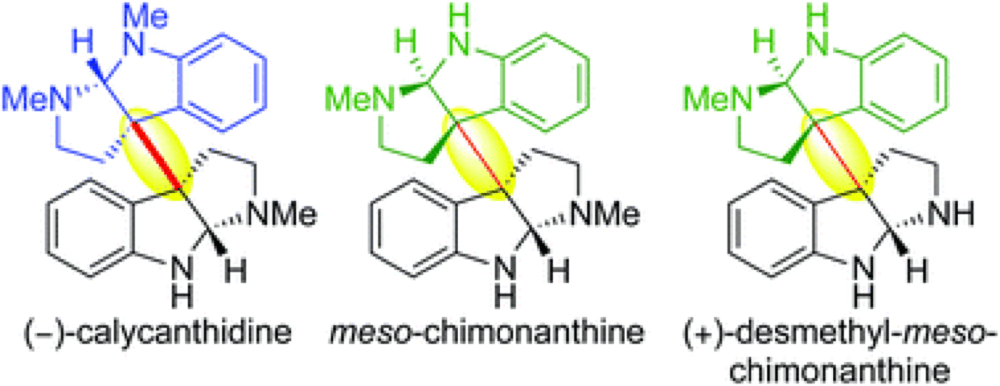
We describe the first application of our methodology for heterodimerization via diazene fragmentation towards the total synthesis of (−)-calycanthidine, meso-chimonanthine, and (+)-desmethyl-meso-chimonanthine. Our syntheses of these alkaloids feature an improved route to C3a-aminocyclotryptamines, an enhanced method for sulfamide synthesis and oxidation, in addition to a late-stage diversification leading to the first enantioselective total synthesis of (+)-desmethyl-meso-chimonanthine and its unambiguous stereochemical assignment.
This versatile strategy for directed assembly of heterodimeric cyclotryptamine alkaloids has broad implications for the controlled synthesis of higher order derivatives with related substructures.