Selective C(sp3)–H Monoarylation Catalyzed by a Covalently Cross-Linked Reverse Micelle-Supported Palladium Catalyst
Caroline B. Hoyt, Li-Chen Lee, Jian He, Jin-Quan Yu, Christopher W. Jones
Advanced Synthesis and Catalysis,
2017, 359, 3611; DOI:10.1002/adsc.201700707
09/2017
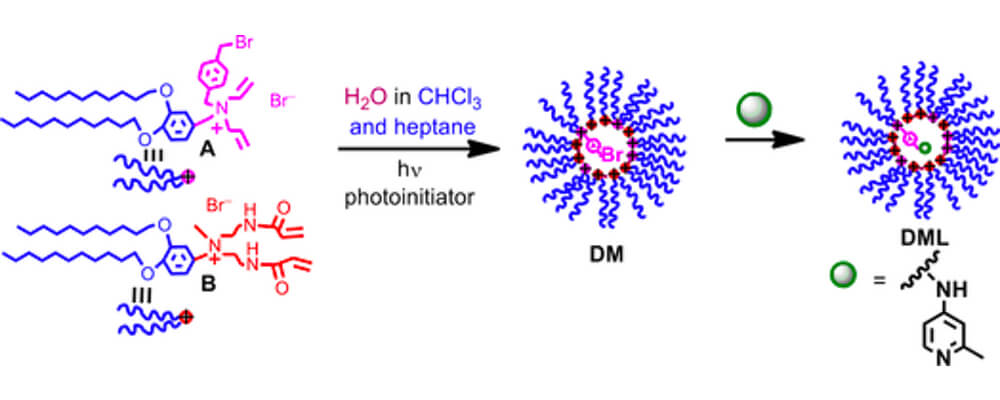
This collaborative report that brings together CCHF researchers from The Scripps Research Institute (TSRI) and the Georgia Institute for Technology, describes the use of micelles as a platform for catalysis in C–H activation chemistry, a technique that improves the recovery of the precious catalyst, changes the solvents and media this reaction can be performed in and offers new avenues for tuning the sterics and electronics of the catalyst microenvironment.
Catalysis is a central tenant of the chemistry being developed within the Center. However, many of the catalysts are based on metals that are either, rare, expensive or unsustainable. In order for these transformations to be applied on a manufacturing scale novel solutions must be sought. This investigation uses a ligand that is contained within a micelle, to which the catalyst can then be attached. Employing the catalyst and reaction development skills of the Yu group at Scripps, who are experts in palladium-catalyzed C–H activation, and the chemical engineering skills of the Jones group at the Georgia Institute of Technology a series of micelle bound catalysts were prepared and their efficiency in known reactions tested.
The reactions were found to proceed with high efficiency, comparing favorably to the non-micelle bound catalysts. The tests also showed that the catalyst could also be recovered and recycled effectively. Future studies will focus both on the design of the micelle around the active species and the sustainability of this process.