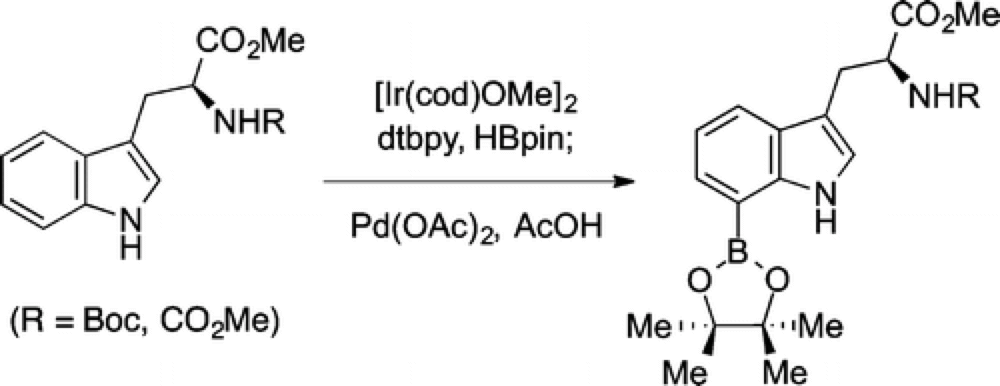
Direct C7 Functionalization of Tryptophan. Synthesis of Methyl (S)-2-((tert-Butoxycarbonyl)amino)-3-(7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indol-3-yl)propanoate
K. Amaike, R. P. Loach, and M. Movassaghi
Organic Synth.,
2015, 92,373-385; 10.15227/orgsyn.092.0373
02/2016
The Movassaghi group recently reported a convenient method for the direct C–H borylation of the C-7 position of a broad variety of indole systems.
Selective functionalization at the C-7 position of indoles has proven particularly problematic, with few methods available that are direct and readily scalable. To demonstrate the application of their new method the Movassaghi group have prepared an Organic Synthesis experimental, with the reaction run efficiently on a 5 gram scale.
This work involved collaborative studies with Kazuma Amaike, a graduate student from the Itami Group in Nagoya, Japan.