Acceleration of reaction in charged microdroplets
Jae Kyoo Lee, Shibdas Banerjee, Hong Gil Nama and Richard N. Zare
Quarterly Review of Biophysics,
2015, 48, 4, 437-444; 10.1017/S0033583515000086
07/2015
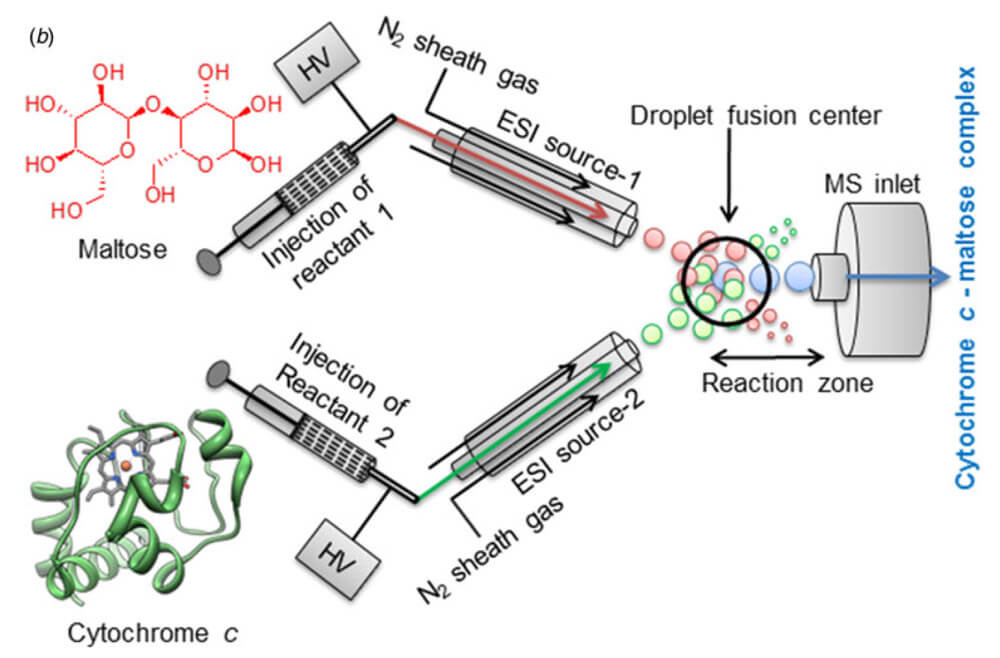
During the course of their studies in the development of ‘microdroplet fusion mass spectrometry’ in order to observe early and transient events in chemical and biochemical processes the Zare group have observed that rate of reaction performed in these microdroplets is significantly greater than that observed in more traditional bulk solutions.
This overview of this emerging technology demonstrates the scope of reaction that can be performed in both charged and non-charged microdroplets, looking at the Pomeranz-Fritsch reaction and the reaction kinetics of cyctochrome C and maltose binding. They explore how performing these reactions in microdroplets impact the rate and pathway of the reaction.
The Zare group observe a remarkable acceleration in the reaction rate when performing both reactions in microdroplets. The effect appears general, regardless of reaction mechanism, including specific covalent and non-specific noncovalent bonding. The causes of this effect are currently under investigation. This new technology is just in its infancy and investigations are underway to explore how this can be applied in new technologies such as C–H Functionalization.