Evolution of Efficient Modular Polyketide Synthases by Homologous Recombination
Joseph A. Chemler, Ashootosh Tripathi, Douglas A. Hansen, Mark O’Neil-Johnson, Russell B. Williams, Courtney Starks, Sung Ryeol Park, and David H. Sherman
J. Am. Chem. Soc.,
2015, 137 (33), pp 10603–10609; 10.1021/jacs.5b04842
07/2015
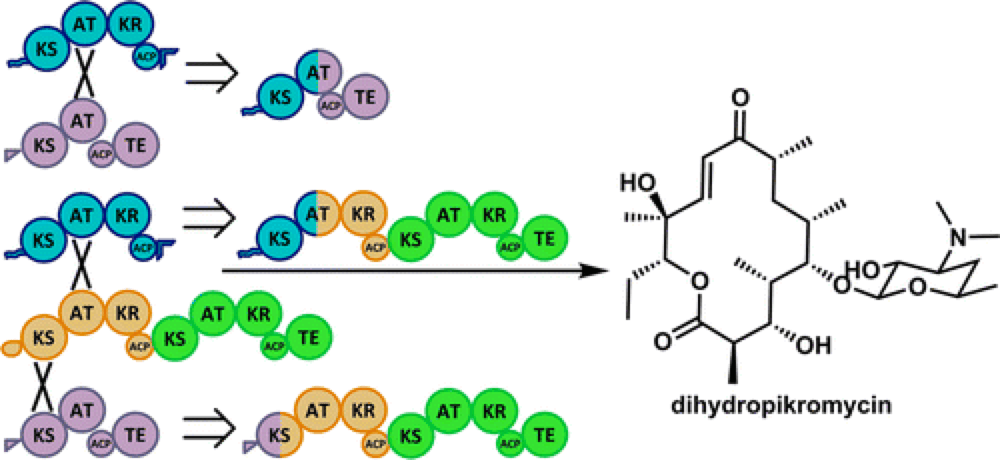
The core molecular frameworks of many complex natural products are produced by multifunctional type 1 polyketide synthase (PKS) enzymes that operate essentially as biosynthetic assembly lines. These assembly lines are modular, which presents the opportunity to customize regions of these megaenzymes in order to build custom biocatalyst systems, put together in a ‘Lego-like’ fashion by inserting, deleting or exchanging native or foreign domains to produce specific targets.
However, previous efforts to build these customized biosynthetic assembly lines have encountered a number of problems that generally resulting in reduced efficiency and low conversion to the desired targets.
This report describes a new approach that is successful in the identification of effective and functional chimeric PKS enzymes for the synthesis of custom macrolactones. This approach is effective not just for the construction of the core of these molecules, but also for the inclusion of enzymes capable of the selective C–H oxidation of the skeleton.