Cobalt catalyzed sp3 C–H amination utilizing aryl azides
Omar Villanueva, Nina Mace Weldy, Simon B. Blakey and Cora E. MacBeth
Chemical Science,
2015, 6, 6672-6675; 10.1039/C5SC01162K
07/2015
The nitrogen atom is found throughout biologically active molecules, including natural products and therapeutic agents, with its electronic structure important in many intermolecular interactions. Thus the direct amination of C–H bonds, in a mild and functional group tolerant manner has been of longstanding interest. There has been significant progress and success in this field with a number of metallonitrene initiated transformations for the intra- and intermolecular introduction of nitrogen in a selective fashion being reported. However, these transformations often require deprotection and subsequent elaboration.
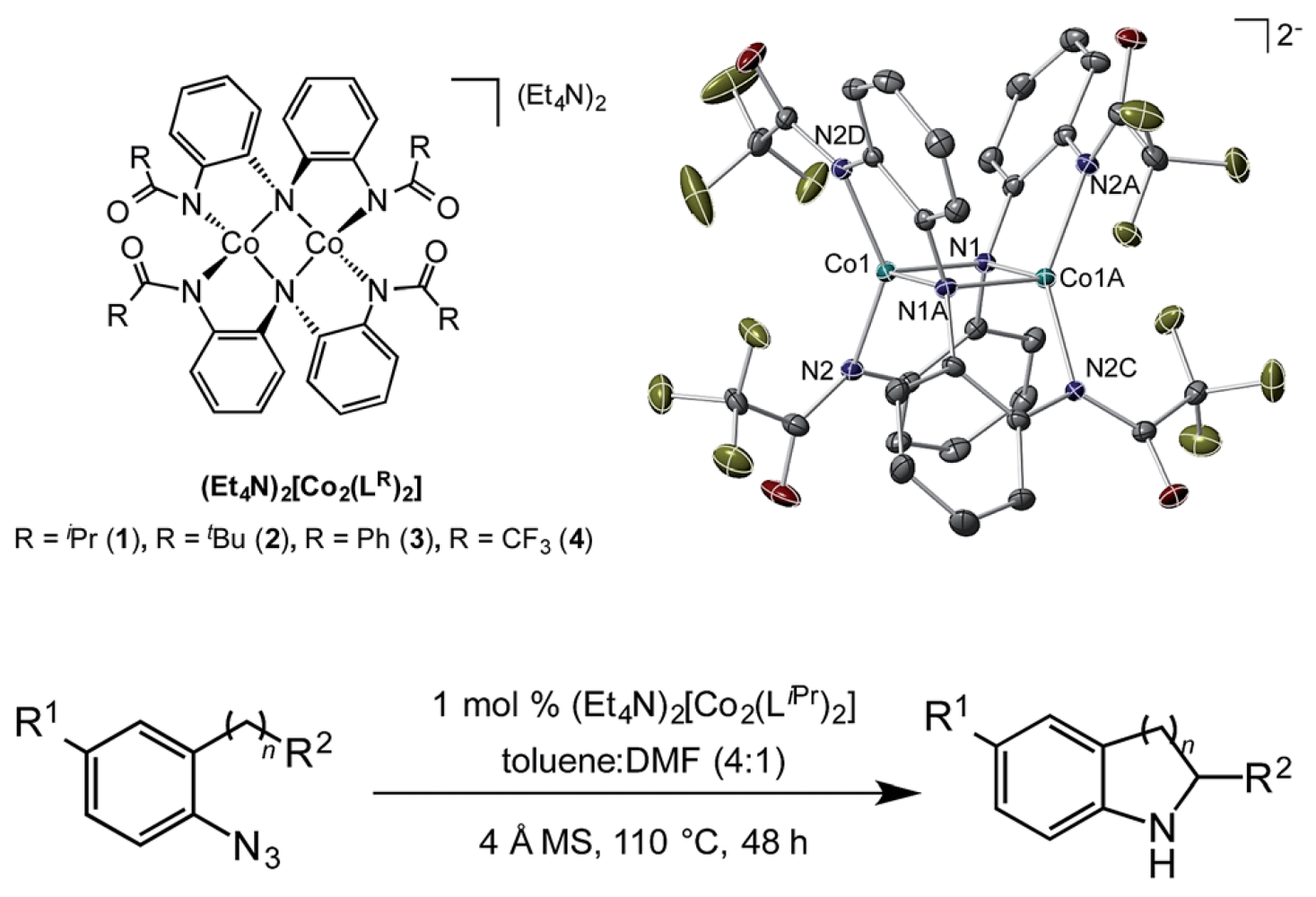
This collaborative report from the MacBeth and Blakey groups builds on some recent work to describe a cobalt catalyzed C–H amination strategy that employs aryl azides as the nitrogen source, furnishing the amination product directly with nitrogen gas as the only byproduct that is tolerant of medicinally relevant heterocycles, and capable of delivering 5-, 6- and 7-membered rings. Previous work in this area has focused on iridium-, rhodium- and iron-catalyzed systems, while the only report of a cobalt-mediated system required stoichiometric amounts of the metal complex.
Recognizing that the nature of the ligand would be critical in these investigations this work built about a previously disclosed modular, tunable and redox-active ligand system that enabled them to tune in the reactivity required for this reaction.