Ligand-Controlled Para-Selective C–H Arylation of Monosubstituted Arenes
Hui Xu, Ming Shang, Hui-Xiong Dai, and Jin-Quan Yu
Organic Letters,
2015, 17 (15), pp 3830–3833; 10.1021/acs.orglett.5b01802
07/2015
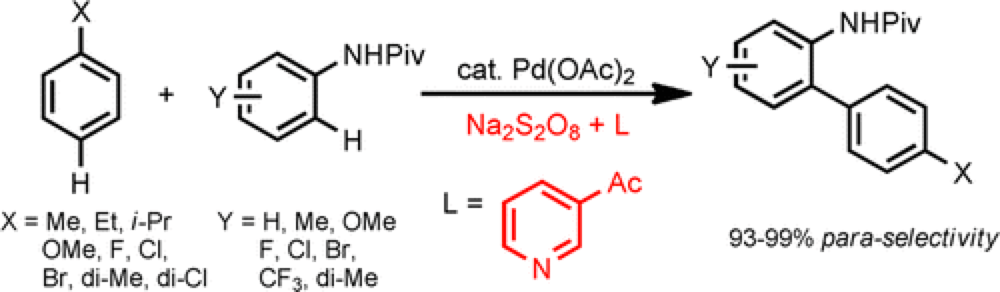
Biaryl scaffolds can be found at the core of many classes of organic molecules, including natural products, therapeutic agents and organic materials. An attractive approach for the synthesis of these systems is through a direct C–H/C–H coupling, removing the need for pre-functionalization and reducing waste by-products.
This collaborative project from the Yu and Dai groups describes, for the first time, a palladium-catalyzed double C–H activation reaction that enables a highly para-selective C–H arylation of monosubstituted arenes. Critical to the success of this reaction is the choice of pyridine-based ligand. Previous reactions have required stoichiometric amounts of F+ oxidant in order to achieve effective para C–H arylation, but through screening of the ligand and effective system has been developed. The reaction is general across a broad scope of electronically and sterically diverse monosubstituted arenes.