Pyridine N-Oxide vs Pyridine Substrates for Rh(III)-Catalyzed Oxidative C–H Bond Functionalization
Sharon R. Neufeldt, Gonzalo Jiménez-Osés, John R. Huckins, Oliver R. Thiel, and K. N. Houk
J. Am. Chem. Soc.,
2015, 137 (31), pp 9843–9854; 10.1021/jacs.5b03535
07/2015
Rh(III)-catalyzed C–H activation has developed into a highly attractive strategy due to the high functional group tolerance of this system resulting in a diverse array of accessible products. Of particular note are annulation strategies on heterocyclic systems that rapidly assemble molecules of therapeutic significance.
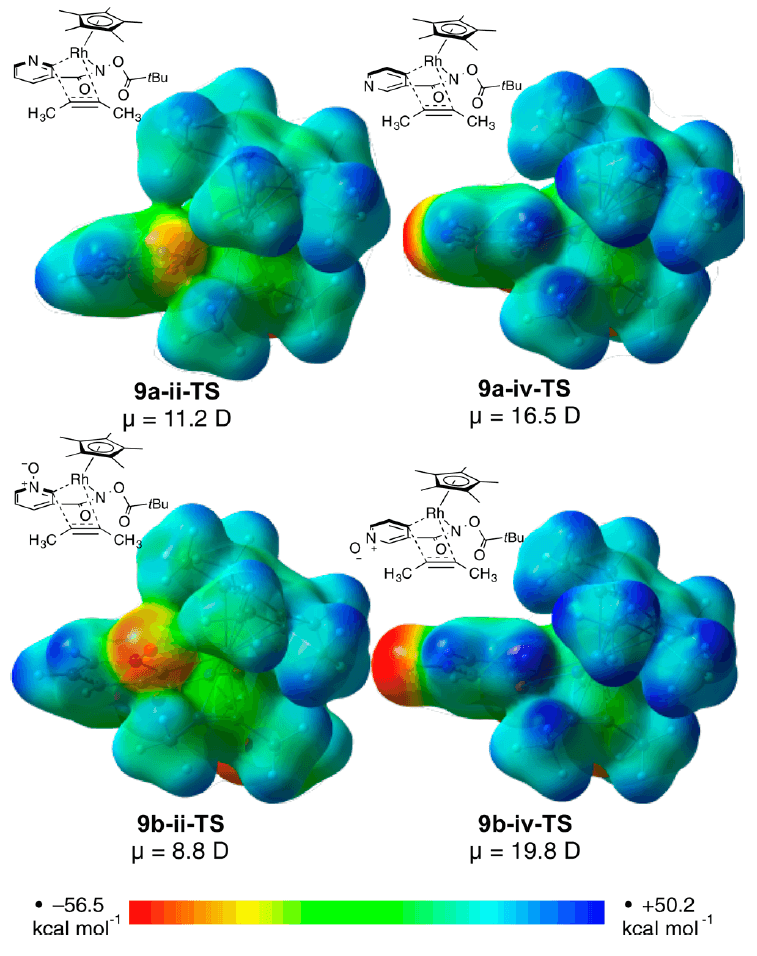
The Houk group has collaborated with researchers from Amgen Process Development to learn more about the origins of selectivity and reactivity in one such system. The mechanism and energetics of the Rh(III)-catalyzed (4+2) annulation of pyridine and pyridine N-oxide substituted with an amide directing group at the 3-position with alkynes was studied using DFT calculations. These studies shed light on the subtleties at play, how the electrostatics of the reagents and nature of the alkyne substrate play a critical roles in determining the product formation.