Highly Active Nickel Catalysts for C–H Functionalization Identified through Analysis of Off-Cycle Intermediates
Alex J. Nett, Wanxiang Zhao, Paul M. Zimmerman and John Montgomery
J. Am. Chem. Soc.,
2015, 137 (24), pp 7636–7639; 10.1021/jacs.5b04548
06/2015
In recent years nickel-catalysis has become widely recognized as a low-cost and sustainable system for the construction of a diverse array of molecular motifs. The use of nickel in C–H functionalization technologies has received particular attention, proving capable of functionalizing sp2 and sp3 C–H bonds.
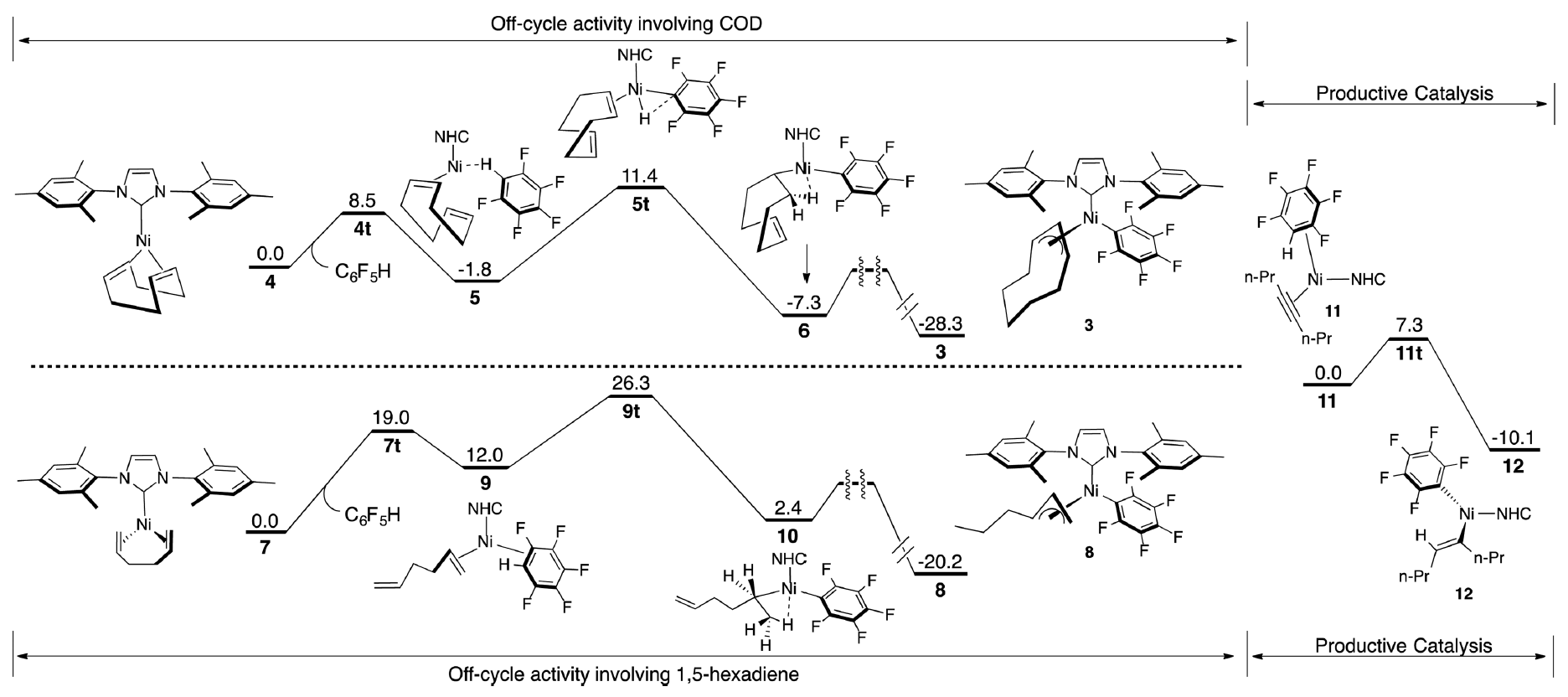
While cost and availability make these systems highly attractive two major drawbacks have been the need for relatively high catalyst loadings and elevated temperature in order for efficient conversion. The Montgomery group, in collaboration with the Zimmerman group, describe a combined experimental and theoretical approach to probe the details nickel-catalysis and the understanding and development of catalysts systems effective at room temperature with significantly increased reactivity profiles.
Through the identification and study of a byproduct it was proposed that Cyclooctadiene, a typical ancillary ligand in nickel-catalyzed reactions, actually facilitated the formation of an off-cycle resting state. Using DFT to study formation of this pi-allyl complex it was proposed that the high temperatures required in nickel-catalyzed C–H functionalization were in order to generate the active catalyst from this resting state. Using these studies as guidance 1,5-hexadiene was incorporated into the Ni(0) complex. Assessment of this complex both theoretically and experimentally found that formation of the off-cycle intermediate was not favored at room temperature, and the catalyst was highly effective in C–H functionalization chemistry.
These findings have significant implications for the design of novel nickel-based C–H functionalization systems and opens the reaction scope for these inexpensive and sustainable catalysts.