Iodoarene-Catalyzed Stereospecific Intramolecular sp3 C–H Amination: Reaction Development and Mechanistic Insights
Chendan Zhu, Yong Liang, Xin Hong, Heqing Sun, Wei-Yin Sun, K. N. Houk , and Zhuangzhi Shi
J. Am. Chem. Soc.,
2015, 137 (24), pp 7564–7567; 10.1021/jacs.5b03488
06/2015
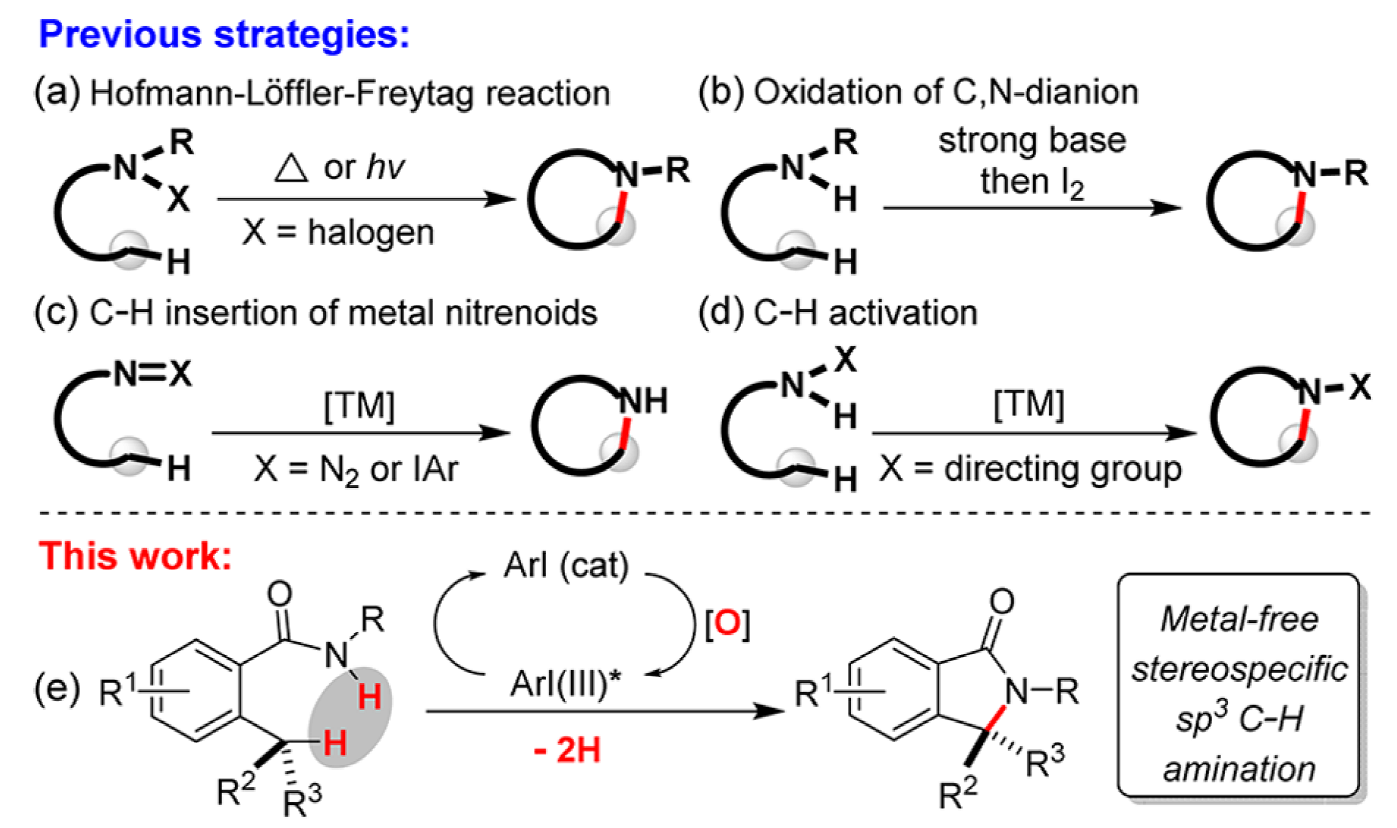
Intramolecular C–H amination has developed into an efficient entry to N-heterocycles, having been demonstrated in the construction of alkaloid natural products and therapeutic agents.
This collaborative study between the Houk and Shi groups explores the mechanism of the first iodoarene-catalyzed intramolecular aliphatic C–H amination, a rapid entry into gamma-lactams. Using DFT calculations the key C–H activation/C–N bonding process was probed, finding that the reaction proceeds via an iodonium cation intermediate through a concerted C–H activation/C–N bonding transition state. The concerted nature of this process allows the construction of chiral quaternary centers.
Iodoarene-catalyzed C–C, C–O and C–N bond forming reactions have recently received significant interest and this extension to C(sp3)–H bonds represents a significant advance.