Enantioselective dirhodium(II)-catalyzed cyclopropanations with trimethylsilylethyl and trichloroethyl aryldiazoacetates
Solymar Negretti, Carolyn M. Cohen, Jane J. Chang, David M. Guptill, Huw M.L. Davies
Tetrahedron
2015, 39, 71, 7415-7420; 10.1016/j.tet.2015.05.045
06/2015
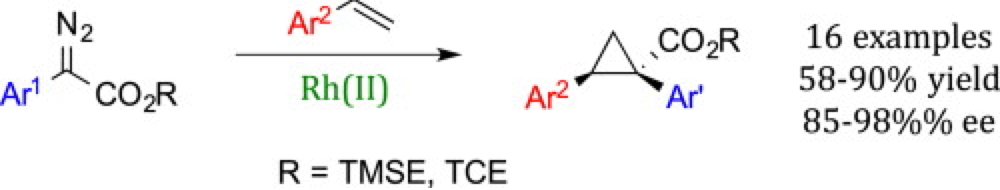
Cyclopropanes are important structural motifs in natural products and medicinal targets as well as versatile intermediates in complex molecule synthesis. Furthermore they play two significant roles in carbene chemistry being investigated by CCHF members; as a testing ground for trend in reactivity and as the basis for the ligand environment of the new generation of chiral dirhodium catalysts.
This report from the Davies group investigates the dirhodium-catalyzed intermolecular asymmetric cyclopropanation of a range of styrenes and alkenes with donor/acceptor carbenes with a particular emphasis of two recently developed labile ester groups, focusing on how the different catalyst structures impact the conversion and selectivity of these sterically and electronically distinct carbenes.
The trends in reactivity highlighted in this report will inform future carbene C–H insertion reaction systems and optimizes access to enantiopure cyclopropane scaffolds for assessment as ligands for dirhodium catalysts.