Mechanism and Stereoselectivity of Directed C(sp3)–H Activation and Arylation Catalyzed by Pd(II) with Pyridine Ligand and Trifluoroacetate
Julong Jiang, Jin-Quan Yu, and Keiji Morokuma
ACS Catalysis
2015, 5 (6), pp 3648–3661; 10.1021/cs501626n
05/2015
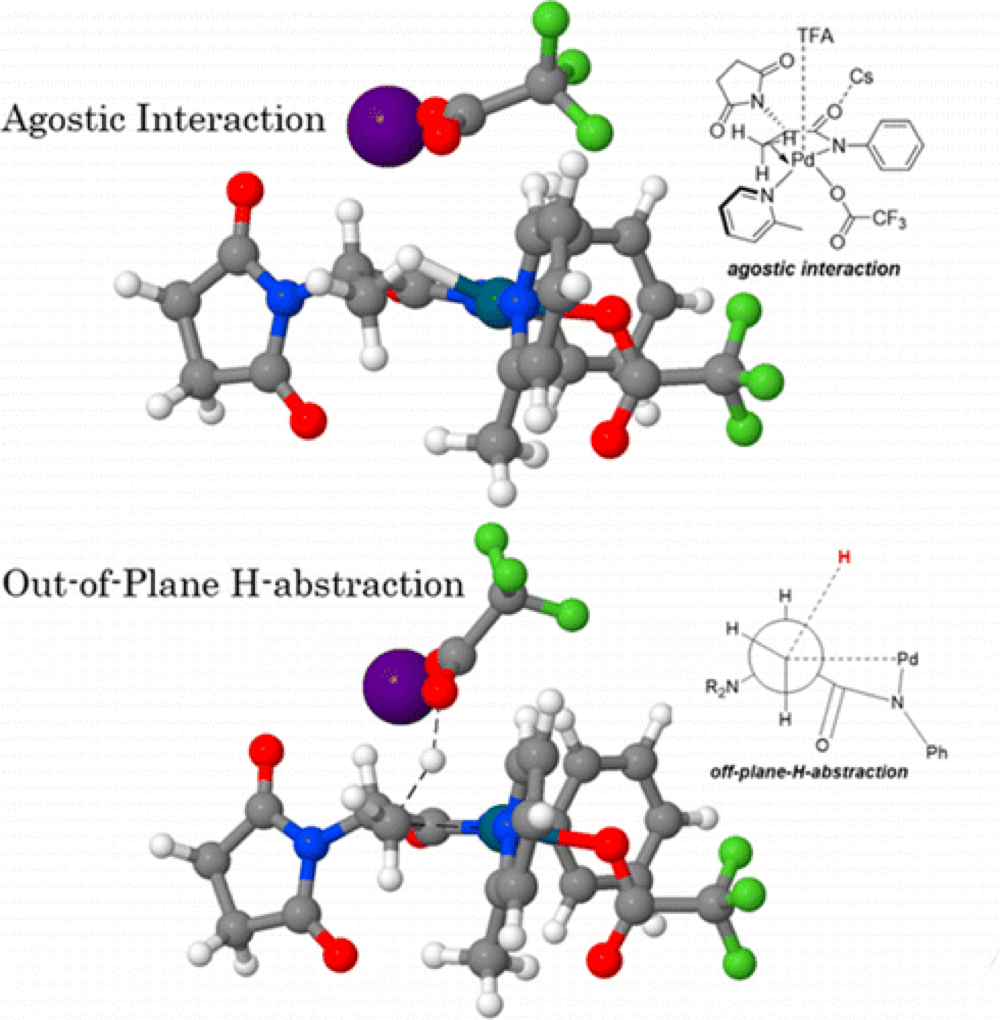
In this collaborative report from the Yu and Morokuma groups the unusual mechanism of the C(sp3)–H bond activation is explored, with particular emphasis on the origins of stereoselectivity.
The DFT studies reveal the formation of a very stable precatalyst that requires activation before the reaction can proceed effectively, including choice of directing group and conditions. The C–H activation was found to proceed through an unconventional deprotonation mechanism, different from the typically invoked CMD pathway. The methyl group was found to be deprotonated through an out-of-plane H-abstraction by an external TFA ligand as opposed to the TFA ligands bound to the metal.
These findings provided the foundation of the studies into the selectivity of the reaction, with the C–H activation determined to be the rate-determining step.