Vicinal Diamination of Alkenes under Rh-Catalysis
David E. Olson , Justin Y. Su , D. Allen Roberts , and J. Du Bois
J. Am. Chem. Soc.,
2014, 136, (39), 13506
09/2014
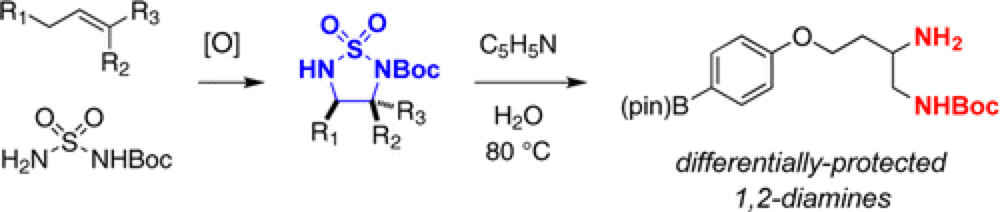
Diamine motifs are common throughout compounds of pharmaceutical interest. The Du Bois group describe a novel route to these priveledged structure.
The synthesis of 1,2-diamines has been achieved through a single-step, tandem sequence involving Rh-catalyzed aziridination followed by NaI-promoted rearrangement to an isomeric cyclic sulfamide. Facile ring opening of these products in hot water and pyridine affords differentially protected vicinal diamines. Demonstration of the utility of this method for the syntheses of (±)-enduracididine and (±)-allo-enduracididine is highlighted.