Ligand-Enabled Cross-Coupling of C(sp3)−H Bonds with Arylsilanes
J. He, R. Takise, H. Fu, and J.-Q. Yu
J. Am. Chem. Soc.,
2015, 137, 4618-4621; 10.1021/jacs.5b00890
03/2015
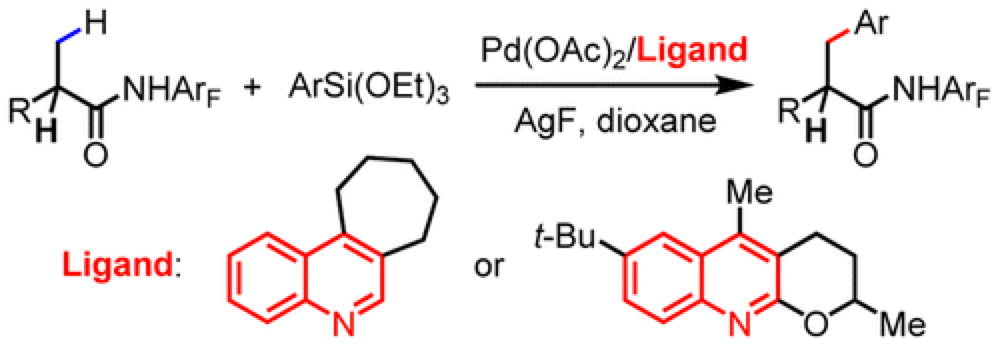
The Yu group report the development of a new ligand system that enables the cross-coupling of beta-C(sp3)–H bonds of carboxylic acid derivatives with arylsilanes.
Cross-coupling reactions have had a huge impact on the way we put together everything from materials to pharmaceuticals. As part of an ongoing program in the Center the Yu group report the development of a reaction equivalent to the Hiyama-type cross-coupling, overcoming a number of obstacles to efficient employ beta-C(sp3)–H as effective coupling partners.
Previous efforts to use this class of substrates had encountered limited substrate scope and problems associated with homo-coupling of substrates. A thorough optimization of the conditions and ligands on the Pd-catalyst employed furnished a protocol that is broad in substrate scope, highly efficient and overcomes many of the previously encountered problems. It was found that the nature of the ligand employed is crucial for an effective reaction.
This new transformation demonstrates an effective, more efficient equivalent of a reaction that has found widespread use in the construction of molecules.
One of the co-authors on this paper, Ryosuke Takise, was a graduate student visitor from the Itami group, part of the ITbM in Japan, at Nagoya University, one of our international partners. He spent three months working at the Scripps Research Institutes.