Late-stage C–H functionalization of complex alkaloids and drug molecules via intermolecular rhodium-carbenoid insertion
Jing He, Lawrence G. Hamann, Huw M. L. Davies and Rohan E. J. Beckwith
Nature Commun.,
2015, 6, 5943; 10.1038/ncomms6943
01/2015
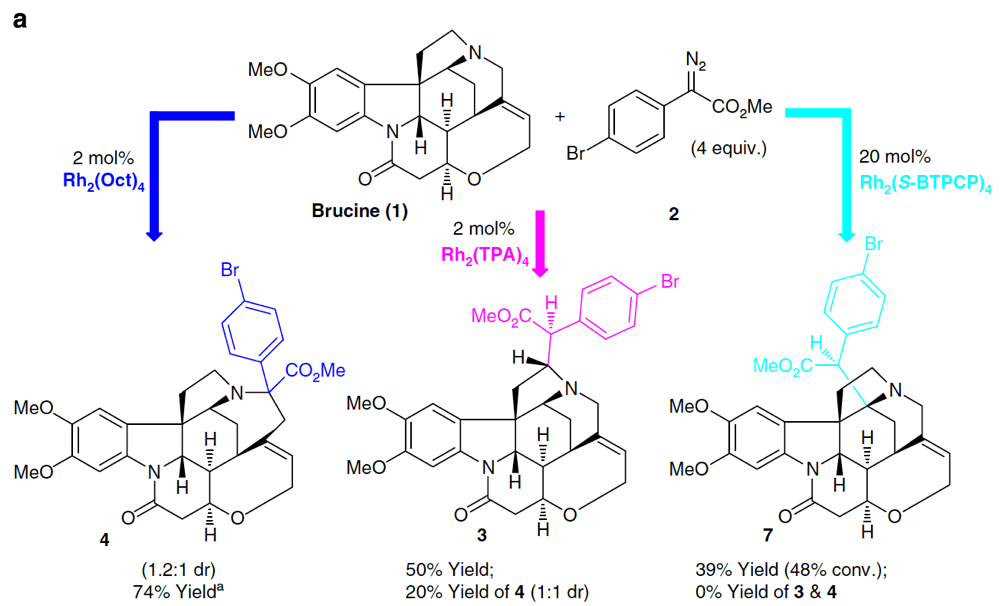
Novartis Institutes for Biomedical Research, in collaboration with Prof. Huw Davies at Emory University, describe their findings on the selective functionalization of C–H bonds in complex alkaloid architectures.
Alkaloids constitute a large family of natural products that possess an array of diverse biological properties. Their complex and challenging structures have been the inspiration and proving ground for many innovations in synthetic chemistry.
This project explores these challenges, including the presence of basic amine motifs and complex skeletons, from the perspective of late-stage C–H functionalization. This report describes the first examples of dirhodium(II)-catalyzed intermolecular C–H insertion into complex natural products containing nucleophilic tertiary amines to generate a C–C bond.
Exquisite control over the site selectivity of this transformation is found possible through catalyst choice. This work performed as a partnership between the CCHF and Novartis Institutes for Biomedical Research demonstrates some of the new challenges and ideas that partnership with companies in the pharmaceutical sector brings to the CCHF.