Pd(II)-Catalyzed meta-C–H Olefination, Arylation, and Acetoxylation of Indolines Using a U-Shaped Template
Guoqiang Yang, Petra Lindovska, Dajian Zhu, Justin Kim, Peng Wang, Ri-Yuan Tang, Mohammad Movassaghi, and Jin-Quan Yu
J. Am. Chem. Soc.,
2014, 136, 10807-10813; 10.1021/ja505737x
07/2014
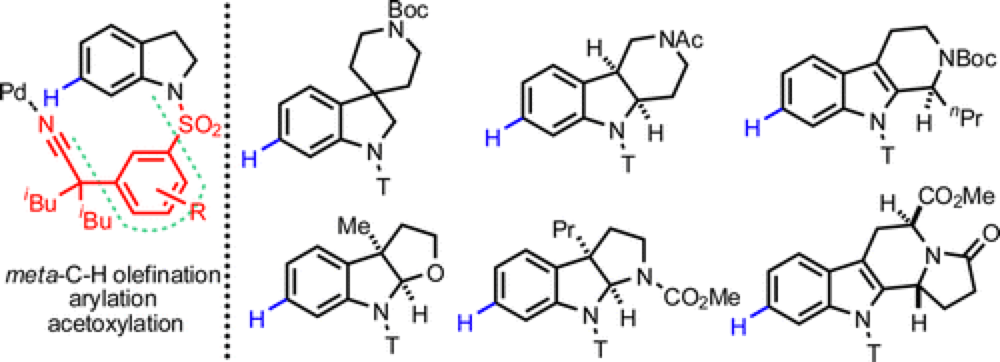
Prof. Jin-Quan Yu’s group from The Scripps Research Institute and Prof. Mo Movassaghi’s group from MIT jointly published a paper in J. Am. Chem. Soc. in which they disclose a new method for the position-selective functionalization of an inert C–H bond in indolines. Three distinct metal-catalyzed transformations were developed to introduce an olefin, an aryl, and an acetoxy groups onto a wide array of indoline substrates. The mild reaction conditions required for these new transformations allowed their application in efficient derivatization of a of complex indoline intermediates, key structural motifs in many Natural products and pharmaceutical molecules.
In the context of the Movassaghi lab’s studies in complex alkaloid total synthesis, a particular unmet challenge in advance–stage functionalization of indoline substructures was identified. Specifically, the Yu group’s recent discoveries in position-selective mild functionalization of complex heterocycles offered an opportunity to provide a general solution to meta-selective derivatization of important indoline substructures. Given the presence of the privileged indoline substructure in many natural products, a general solution for this transformation would be of broad interest and could provide new and enabling synthetic disconnections. Joint efforts led by postdoctoral researcher Dr. Guo-Qiang Yang (Yu lab) and graduate student Petra Lindovska (Movassaghi lab) in combination with contributions from other members of the two research groups were launched between the Movassaghi and Yu labs to find a solution for region-selective functionalization of indoline and related heterocycles. Through extensive rational design and experimentation, they found that a new template attached to the indolyl nitrogen could direct Pd catalyst to reach the remote C-7 position and perform the C-H activation. This process was crucially accelerated by a mono-protected amino acid ligand (MPAA).
Currently, the two groups are developing and applying other transformations based on this remote selectivity to allow more rapid and efficient entry to a range of complex alkaloids.