Role of Sterically Demanding Chiral Dirhodium Catalysts in Site-Selective C–H Functionalization of Activated Primary C–H Bonds
Changming Qin and Huw M. L. Davies
J. Am. Chem. Soc.,
2014, 136, 9792-9796; 10.1021/ja504797x
06/2014
Atlanta GA – Researchers at Emory University, led by Prof. Huw Davies, a synthetic organic chemist, have designed a new family of rhodium(II)-catalysts that offers a level of control that will significantly expand the scope of C–H functionalization, a technique set to revolutionize the way we put together organic molecules.
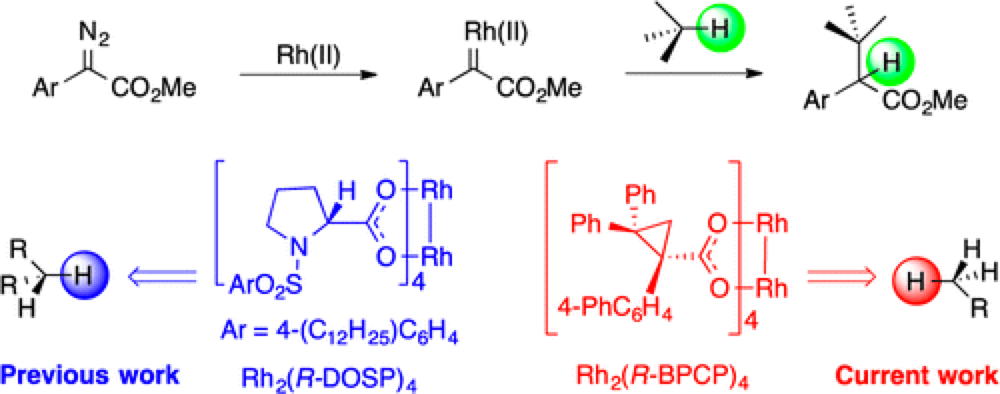
Metallo-Carbenes have been demonstrated to be highly effective reagents for selective C–H insertion and C–C bond formation. The major challenge in this field is selecting a particular C–H bond from the plethora commonly present in an organic molecule. The site of reaction is determined by a fine balance between the steric and electronic properties of both the substrate and catalyst. More established rhodium(II)-catalysts have shown a predilection for electron-rich secondary C–H bonds. However a new class of rhodium(II)-catalysts, the ligands for which are based on cyclopropanes, themselves synthesized using rhodium(II)-carbenoids, demonstrate a very different selectivity profile. Reaction with these new catalysts no longer reacts preferentially at secondary C–H bonds, instead favoring the more sterically accessible, but less electronically stabilized primary C–H bonds.
While this work is still at an early stage, this level of control takes the field one step closer to realizing a key long term goal; development of a ‘toolbox’ of catalysts that will allow the selective reaction of different C–H bonds within an organic molecule. “This is a major advance for our group in our quest to control which C-H bond is functionalized in a complex molecule by simply choosing the appropriate catalyst”, stated Huw Davies, the corresponding author of this publication.