Role of Ortho-Substituents on Rhodium-Catalyzed Asymmetric Synthesis of β-Lactones by Intramolecular C–H Insertions of Aryldiazoacetates
Liangbing Fu, Hengbin Wang, and Huw M. L. Davies
Organic Letters,
2014, 16, (11), 3036-3039; 10.1021/ol5011505
05/2014
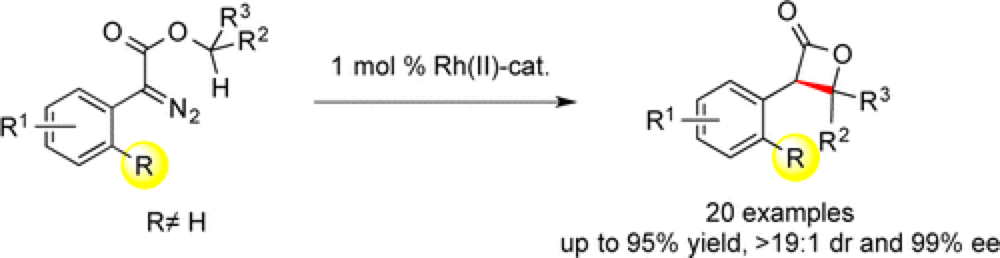
The Davies group report a novel synthesis of beta-lactones via a dirhodium-catalyzed intramolecular C–H insertion.
The incorporation of a halo- or trifluoromethyl-substitutent at the ortho position on the aryl group of a donor/acceptor carbene effectively shuts down many of the deleterious intermolecular side reactions normally associated with these systems, enabling the intramolecular reaction profile.
Beta-lactones are structural motifs of significant pharmaceutical interest due to their biological activity and this represents a streamlined entry into these valuable molecules.