Distortion/Interaction Analysis Reveals the Origins of Selectivities in Iridium-Catalyzed C–H Borylation of Substituted Arenes and 5-Membered Heterocycles
Aaron G Green, Peng Liu, Craig A Merlic, and K. N. Houk
J. Am. Chem. Soc.,
2014, 136, 4575-4583; 10.1021/ja411699u
02/2014
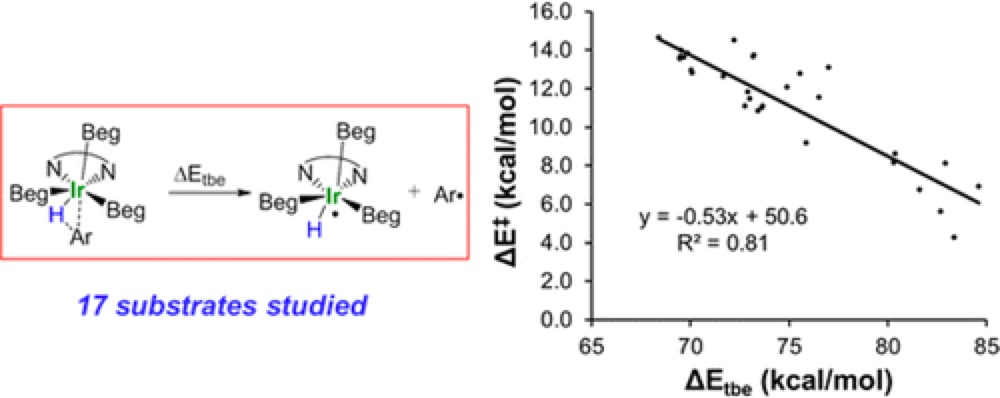
The Houk group have collaborated with the Merlic group from UCLA to investigate the mechanistic details of iridium-catalyzed C–H borylation of disubstituted arenes and heteroarenes.
The DFT calculations reveal that the transition states for C–H oxidative addition occur very late in the reaction pathway, resembling the aryl iridium hydride intermediate with a formed Ir–C bond.
This finding suggests that the regioselectivity is mainly controlled by differences in the interaction energies between the Ir-catalyst and the arene carbon.
These finding will be important in expanding the scope of this reaction and designing the next generation of catalysts.