Controllable Direct Arylation: Fast Route to Symmetrical and Unsymmetrical 4,7-Diaryl-5,6-difluoro-2,1,3-benzothiadiazole Derivatives for Organic Optoelectronic Materials
Junxiang Zhang, Wayne Chen, Anthony J. Rojas, Evgheni V. Jucov, Tatiana V. Timofeeva, Timothy C. Parker, Stephen Barlow, and Seth R. Marder
J. Am. Chem. Soc.,
2013, 135, 16376-16379; 10.1021/ja4095878
10/2013
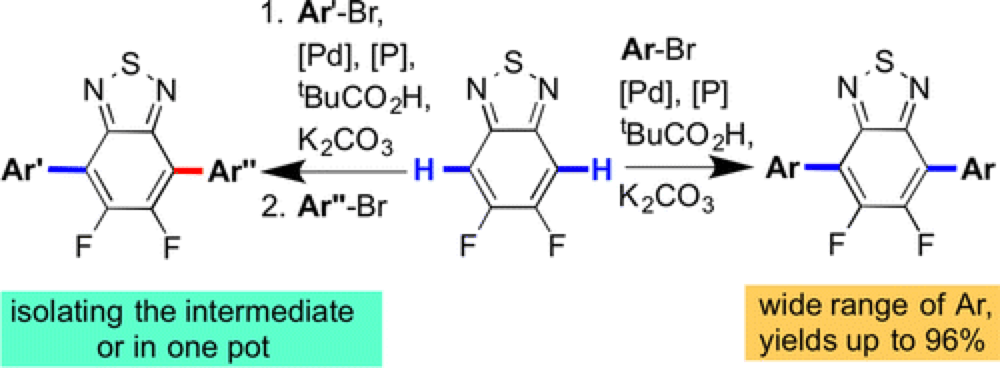
The Marder group report a novel technique for the rapid functionalization of important building blocks for electron conducting polymers using palladium-catalyzed C–H functionalization.
Using this technique arylation in the 4- and 7-positions of the 2,1,3-benzothiadiazole (BT) and its monofluoro- (MFBT) and difluoro-(DFBT) derivatives by (hetero)aryl bromides was possible in with good conversion. Furthermore, arylation is possible in a sequential fashion, providing ready access to differentially substituted derivatives.