Unprecedented Dearomatized Spirocyclopropane in a Sequential Rh(III)-catalyzed C-H Activation and Rearrangement Reaction
Xiaoming Wang, Yingzi Li, Tobias Knecht, Constantin Daniliuc, Ken Houk, Frank Glorius
Angew. Chem. Int. Ed.,
2018, 57, 5520; DOI:10.1002/anie.201800803
02/2018
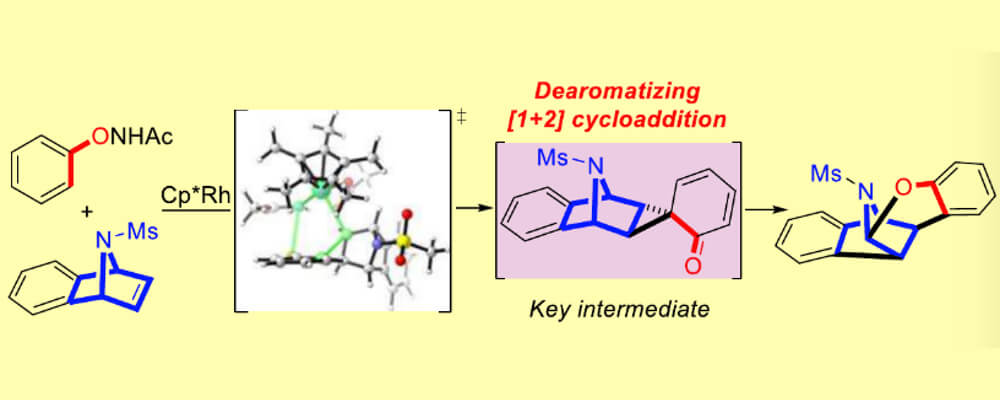
In the course of a study of a Cp*Rh(III)-catalyzed C–H activation, an unusual dearomatized spirocyclopropane intermediate was discovered. It arises from a sequential Cp*Rh(III)-catalyzed C–H activation and Wagner–Meerwein-type rearrangement reaction.
By a combination of the experimental work of the Glorious group in Münster, and the computational study with DFT by Houk’s group at UCLA (and a CCHF member), the mechanisms of oxidative O–N bond cleavage and HOAc involvement were uncovered in this study. Furthermore, a Cp*Rh(III)-catalyzed dearomatization reaction of N-(naphthalen-1-yloxy)acetamide with strained olefins was developed, affording a variety of spirocyclopropanes.