Evolving artificial metalloenzymes via random mutagenesis
Hao Yang, Alan M. Swartz, Hyun June Park, Poonam Srivastava, Ken Ellis-Guardiola, David M. Upp, Gihoon Lee, Ketaki Belsare, Yifan Gu, Chen Zhang, Raymond E. Moellering and Jared C. Lewis
Nature Chemistry,
2018, 10, 318; DOI:10.1038/nchem.2927
01/2018
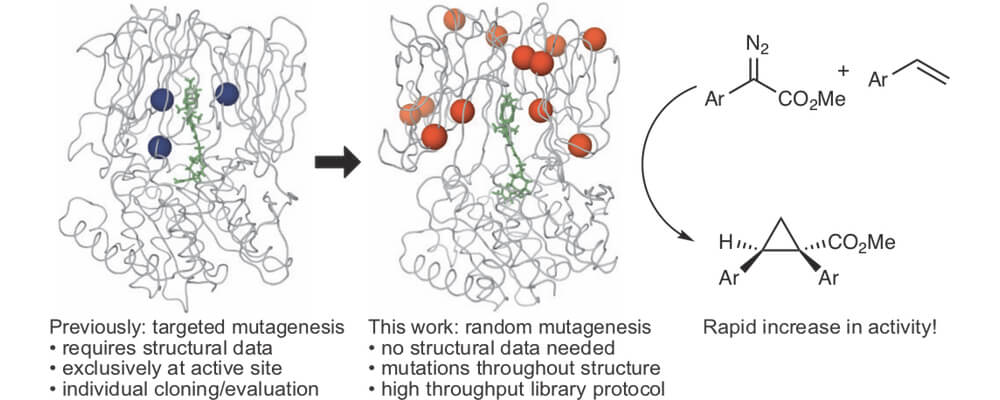
In this work, the Lewis group demonstrate that artificial metalloenzymes, protein scaffolds containing an unnatural metal cofactor, can be enhanced through random evolutionary approaches similar to natural enzymes.
One of the most synthetically important features of natural and unnatural enzymatic catalysts is their capacity for evolution. Natural enzymes have been evolved in the laboratory to meet a variety of important synthetic characteristics, such as improved efficiency, organic solvent tolerance and selectivity. We set out to show that artificial metalloenzymes can be improved in the same way. First, an iterative evolution protocol was developed that enabled screening of a large number of mutants. Using this method over multiple rounds enabled identification of a much improved cyclopropanase.
We use a dirhodium tetracarboxylate cofactor that catalyzes numerous reactions, including C-H functionalization. Cyclopropanation of alkenes is used as a model reaction in order to develop a directed evolution protocol that can be used to improve other reaction types.
Author: David Upp