Pd-Catalyzed Ortho C–H Hydroxylation of Benzaldehydes Using a Transient Directing Group
Xiao-Yang Chen, Seyma Ozturk, and Erik J. Sorensen
Organic Letters,
2017, 19, (23), 6280; DOI:10.1021/acs.orglett.7b02906
11/2017
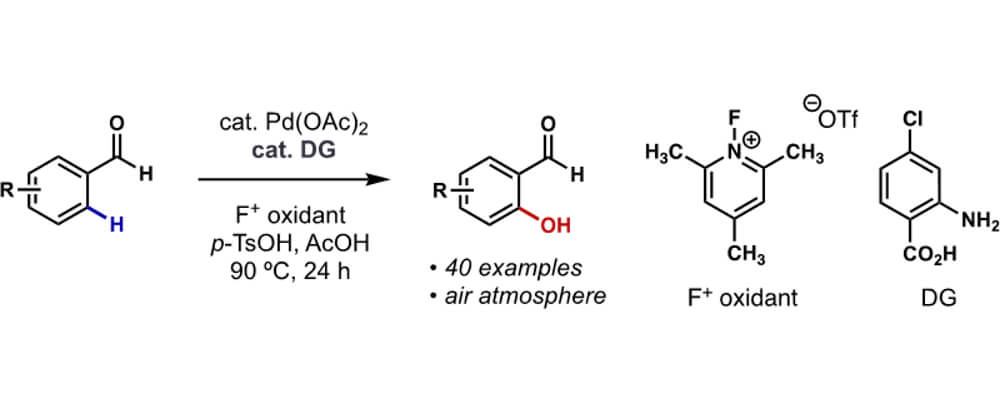
Direct oxidation of the ortho C-H of benzaldehydes is an appealing transformation because the products, salicylaldehydes, can be easily derivatized to a number of pharmaceutically important compounds such as catechols, benzofurans and coumarins. Unfortunately, most of the current catalytic methods are incompatible with such purpose. For one thing, the weak coordinating ability of aldehydes prevents many transition metals from undergoing chelation-assisted C-H metalation at the ortho position.
For another, many common oxidative conditions would either cause over-oxidation of the aldehydes or decomposition of the transient directing groups. Herein, I demonstrated that by using an appropriate F+ reagent as the bystanding oxidant for Pd(II) and p-TsOH as the oxygen nucleophile for the Pd(IV) intermediate,4 the desired ortho-hydroxylated benzaldehydes could be furnished in moderate to good yields. By virtue of an anthranilic acid-type transient directing group, the reaction circumvented the weak coordinating power of the formyl group and was compatible with a wide variety of substrates.
Author: Xiaoyang Chen