Mechanistic Basis for Efficient, Site-Selective, Aerobic Catalytic Turnover in Pd-Catalyzed C–H Imidoylation of Heterocycle-Containing Molecules
Stephen J. Tereniak and Shannon S. Stahl
J. Am. Chem. Soc.,
2017, 139, 14533; DOI:10.1021/jacs.7b07359
09/2017
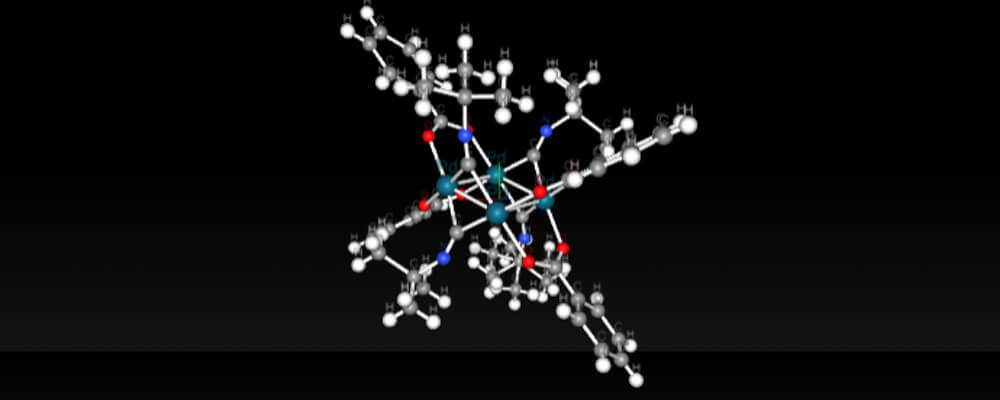
This study from the Stahl Group, at the University of Wisconsin-Madison, explores some of the mechanistic features of aerobic oxidation reactions.
Oxidation reactions are a fundamental class of transformations, not just in organic chemistry, but across the chemical sciences. In order to perform an oxidation reaction, you need an oxidant, a substance that has the ability to oxidize other substances. There are many oxidants used by synthetic organic chemists, but arguably the most available and environmentally friendly oxidant is oxygen itself. This mechanistic study explores some of the key questions in using oxygen as an oxidant.
Using a class of heterocycles known as iminoisoindolinones three questions were asked: (1) Why does the reaction give better yields with ambient air than with pure O2? (2) Why does the reaction occur exclusively at a C–H bond next to the N-methoxyamide group instead of at C–H bonds next to other directing groups? (3) Why are palladium(0) catalysts more effective than palladium(II) catalysts?
The answers to these questions, which are outlined in the paper, will help facilitate the design of new and improved aerobic oxidative reactions.