Enantioselective Total Synthesis of Nigelladine A via Late-Stage C–H Oxidation Enabled by an Engineered P450 Enzyme
Steven A. Loskot, David K. Romney, Frances H. Arnold, and Brian M. Stoltz
J. Am. Chem. Soc.,
2017, 139, (30), 10196; DOI:10.1021/jacs.7b05196
07/2017
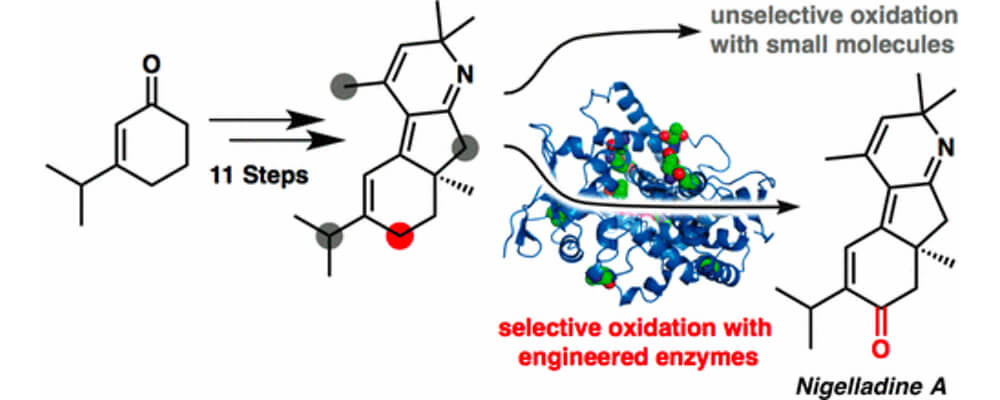
An enantioselective total synthesis of the norditerpenoid alkaloid nigelladine A is described, that brought together the Stoltz and Arnold groups.
Strategically, the synthesis relies on a late-stage C–H oxidation of an advanced intermediate. While traditional chemical methods failed to deliver the desired outcome, an engineered cytochrome P450 enzyme was employed to effect a chemo- and regioselective allylic C–H oxidation in the presence of four oxidizable positions. The enzyme variant was readily identified from a focused library of three enzymes, allowing for completion of the synthesis without the need for extensive screening.
Interestingly David Romney was an undergraduate student in the Stoltz lab, a graduate student at Yale in the Miller lab (a member of the CCHF Scientific Advisory Board), and then went on to work as a postdoctoral research fellow in the Arnold lab.