Copper-Mediated Late-Stage Functionalization of Heterocycle-Containing Molecules
Ming Shang, Ming-Ming Wang, Tyler G. Saint-Denis, Ming-Hong Li, Hui-Xiong Dai, Jin-Quan Yu
Angew. Chem. Int. Ed.,
2017, 56, 19, 5317-5321; DOI: 10.1002/anie.201611287
04/2017
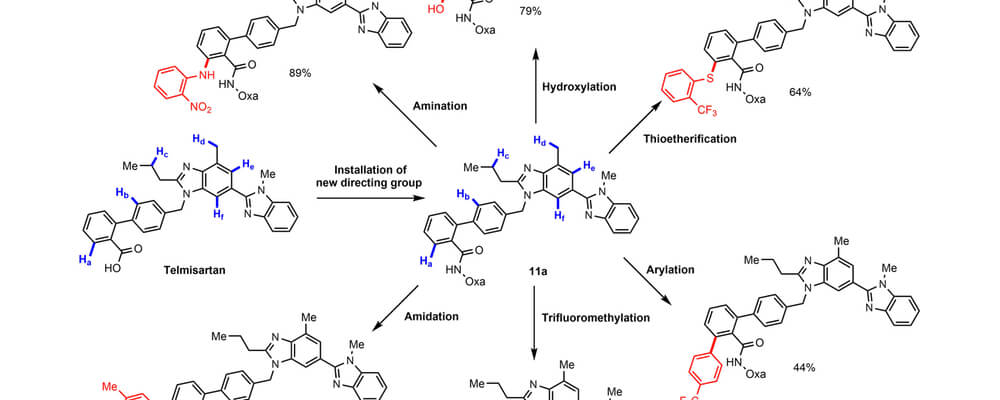
One long-standing issue in directed C−H functionalization is that heteroatoms present in molecules substrates may bind preferentially to a transition-metal catalyst rather than to the desired directing group. This competitive binding has been one of the keys factors that has hindered the application of C−H functionalization in late-stage heterocycle drug discovery.
Reported here, by the Yu and Dai groups, is the use of an oxazoline-based directing group capable of overriding the poisoning effect of a wide range of heterocycle substrates. The potential use of this directing group in pharmaceutical drug discovery is illustrated by diversification of Telmisartan (an antagonist for the angiotensin II receptor) through copper-mediated C−H amination, hydroxylation, thiolation, arylation, and trifluoromethylation.