Catalyst-Controlled Nitrene Transfer by Tuning Metal:Ligand Ratios: Insight into the Mechanisms of Chemoselectivity
Cale Weatherly, Juliet M. Alderson, John F. Berry, Jason E. Hein, and Jennifer M. Schomaker
Organometallics,
2017, 36 (8), 1649-1661; DOI: 10.1021/acs.organomet.7b00190
04/2017
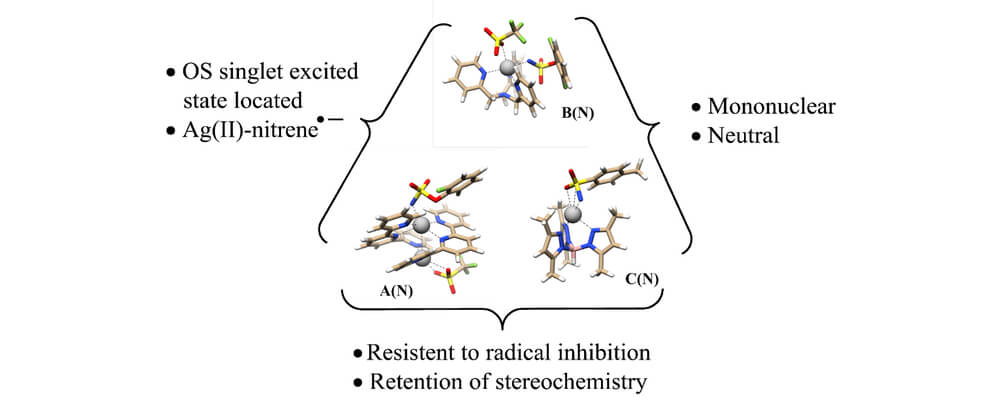
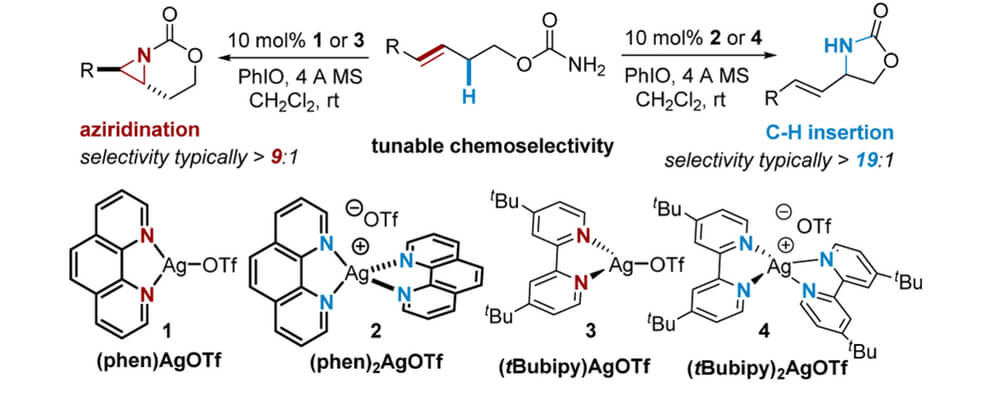
Catalyst-controlled, selective nitrene transfer is often challenging when both C–H and C═C bonds are present in a substrate. Interestingly, a simple change in the Ag(I):L ratio (L = bidentate N,N-donor ligand) enables tunable, chemoselective nitrene transfer that favors either C═C bond aziridination using an ∼1:1 Ag:L ratio (AgLOTf) or insertion into a C–H bond when the Ag:L ratio in the catalyst is 1:2 (AgL2OTf).
In this article, that brings together the Berry, Schomaker and Hien groups in a collaborative investigation, mechanistic studies, coupled with kinetic profiling of the entire reaction course, are employed to examine the reasons for this unusual behavior. Steady-state kinetics were found to be similar for both AgLOTf and AgL2OTf; both complexes yield electronically similar reactive intermediates that engage in nitrene transfer involving formation of a short-lived radical intermediate and barrierless radical recombination.
Taken together, experimental and computational studies point to two effects that control tunable chemoselectivity: suppression of aziridination as the steric congestion around the silver center is increased in AgL2OTf and a decrease in the rate of C–H insertion with AgLOTf in comparison to AgL2OTf. The observation that the sterics of Ag catalysts can be varied, with minor effects on the electronic features of the putative nitrene, has important implications for the development of other silver catalysts that enable tunable, site-selective C–H bond aminations.