Synthesis of Fluorenones from Benzaldehydes and Aryl Iodides: Dual C–H Functionalizations Using a Transient Directing Group
Xiao-Yang Chen, Seyma Ozturk and Erik J. Sorensen
Organic Letters
2017, 19, (5), 1140; DOI: 10.1021/acs.orglett.7b00161
02/2017
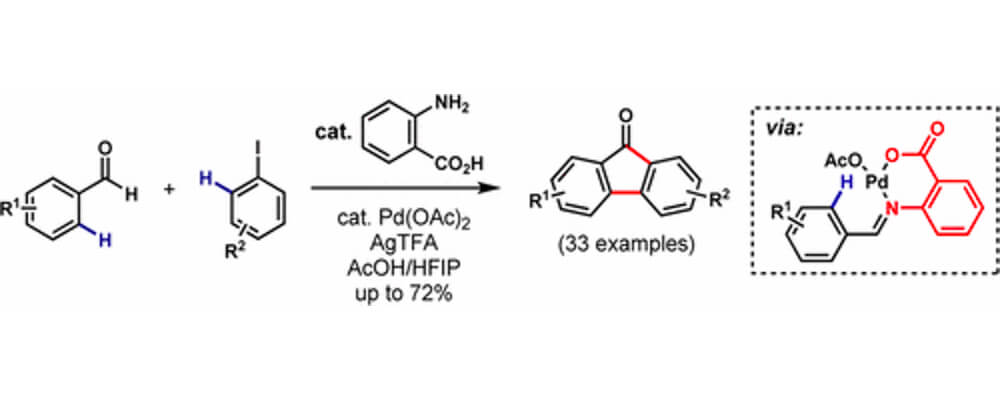
The first synthesis of substituted fluorenones directly from benzaldehydes and aryl iodides via a Pd(II)-catalyzed C(sp2)–H functionalization cascade is reported. Featuring anthranilic acid as an inexpensive transient directing group, the process is compatible with a variety of benzaldehydes and aryl iodides. A three-step synthesis of the antiviral drug Tilorone was completed in an excellent overall yield (40%), demonstrating the utility of this method.