Room-temperature carbon–sulfur bond formation from Ni(II) s-aryl complex via cleavage of the S–S bond of disulfide moieties
Ken Okamoto, Jeremy B. Housekeeper, Christine K. Luscombe
App. Organomett. Chem.,
2013, 27, (11), 639-643; 10.1002/aoc.2975
05/2013
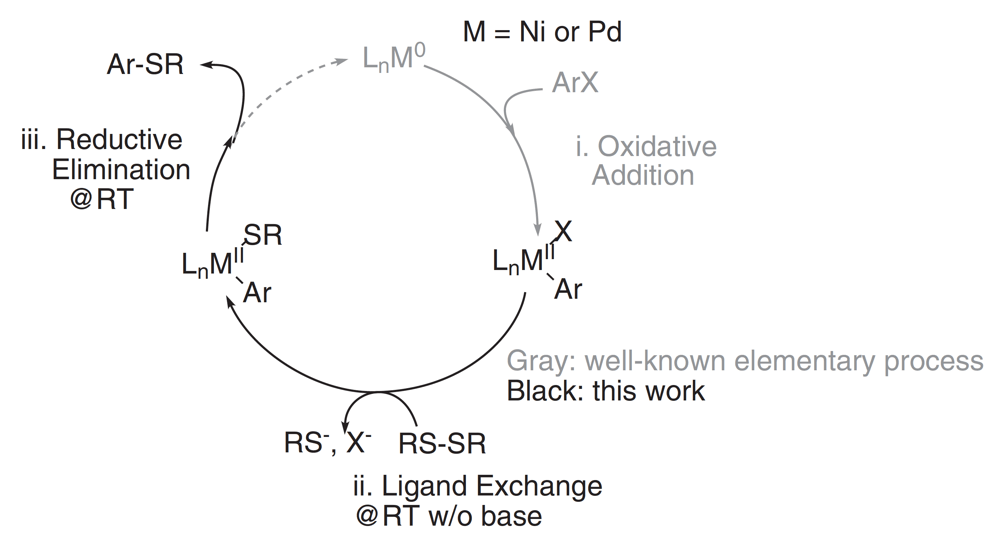
The formation of sp2 C-S bonds is an important transformation in organic chemistry, with particular significance in the Materials Sciences where functional aryl sulfides are key components of organic electronic polymers. Previous methods relied upon palladium and nickel catalyzed transformations that required forcing reaction conditions and elevated temperatures when using disulfides, the favored sulfuration agent. This report by the Luscombe group describes some informed catalyst design in order to overcome these barriers and deliver a room-temperature, mild C–S bond formation via C–H Activation.
It was found that pre-treatment of the disulfide with tri-phenylphosphine allowed facile cleavage of the S-S bond and catalyzed the formation of the desired C-S bond, a significant advance for sulfur donating agents.