Catalytic Anti-Markovnikov Transformations of Hindered Terminal Alkenes Enabled by Aldehyde-Selective Wacker-Type Oxidation
Kelly E. Kim, Jiaming Li, Robert H. Grubbs, Brian M. Stoltz
J. Am. Chem. Soc.,
2016, 138 (40), pp 13179–13182; DOI: 10.1021/jacs.6b08788
09/2016
An extremely versatile and robust transformation, the Tsuji–Wacker reaction is formally among the oldest known methods for C–H oxidation and has found applications in the production of commodity chemicals and the synthesis of complex molecules. The synthetic utility of the Tsuji–Wacker oxidation stems from its efficiency and broad functional group compatibility, and modifications to the original conditions have further expanded its applications.
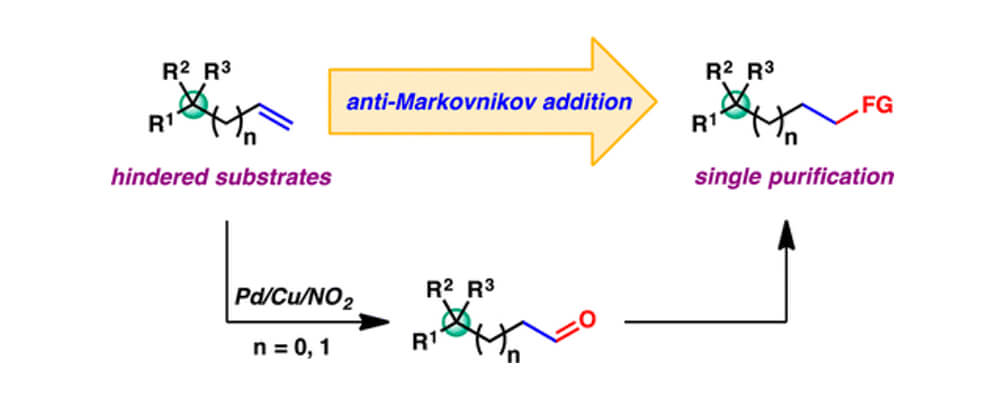
A notable modification pioneered by the Grubbs research group entails the reversal in regioselectivity from the traditional Tsuji–Wacker reaction, permitting selective formation of aldehyde products through use of a nitrite co-catalyst. Given their inherent reactivity, aldehydes can serve as versatile intermediates in the preparation of various other functional groups. This work discloses novel strategies toward the functionalization of sterically hindered terminal olefins, common intermediates in complex molecule synthesis, enabled by the nitrite-modified Tsuji–Wacker reaction.
Alkenes bearing quaternary carbons at the allylic or homoallylic position are demonstrated to be viable substrates for aldehyde-selective Tsuji–Wacker oxidation, which tolerates a wide range of functional groups. The crude aldehyde products are transformed further, resulting in direct conversion of hindered terminal alkenes to various other synthetically useful functional groups in an anti-Markovnikov fashion. Formal transformations accessible through this strategy include hydroamination, hydration, homologation, and indole synthesis.
Author: Kelly Kim