Concise Total Syntheses of (+)-Haplocidine and (+)-Haplocine via Late-Stage Oxidation of (+)-Fendleridine Derivatives
Kolby L. White and Mohammad Movassaghi
J. Am. Chem. Soc.,
2016, 138 (35), pp 11383–11389; DOI: 10.1021/jacs.6b07623
08/2016
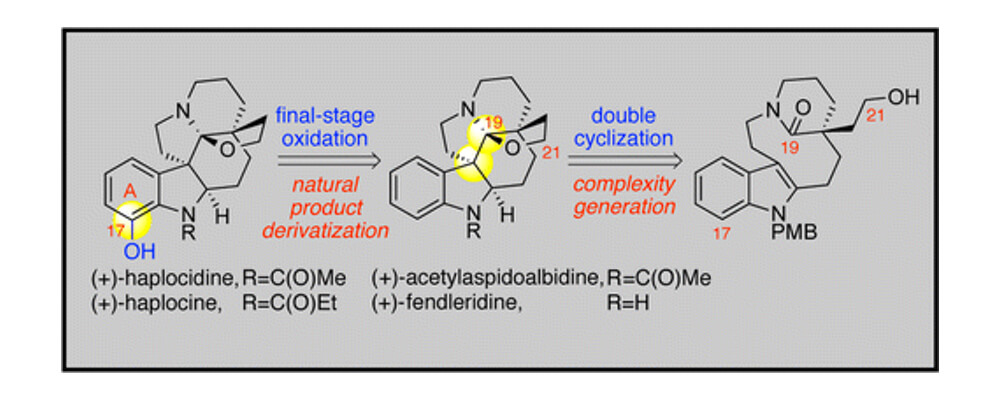
The Movassaghi group describe a project that combines their established expertize in the synthesis of natural products with techniques developed in the Yu lab for the oxidation of C(sp2)–H bonds.
Using a double cyclization strategy the Movassaghi group rapidly stitched together the carbon framework of the aspidosperma natural product core. Using the understanding developed around the Yu groups palladium-catalyzed C–H oxidation, a late-stage amide directed ortho-oxidation of indoline amides enabled access to a range of natural products from a single C–H intermediate.