Pd-Catalyzed α-Selective C–H Functionalization of Olefins: En Route to 4-Imino-β-Lactams
Wei-Jun Kong, Yue-Jin Liu, Hui Xu, Yan-Qiao Chen, Hui-Xiong Dai, and Jin-Quan Yu
J. Am. Chem. Soc.,
2016, 138, (7), 2146-2149; 10.1021/jacs.5b13353
02/2016
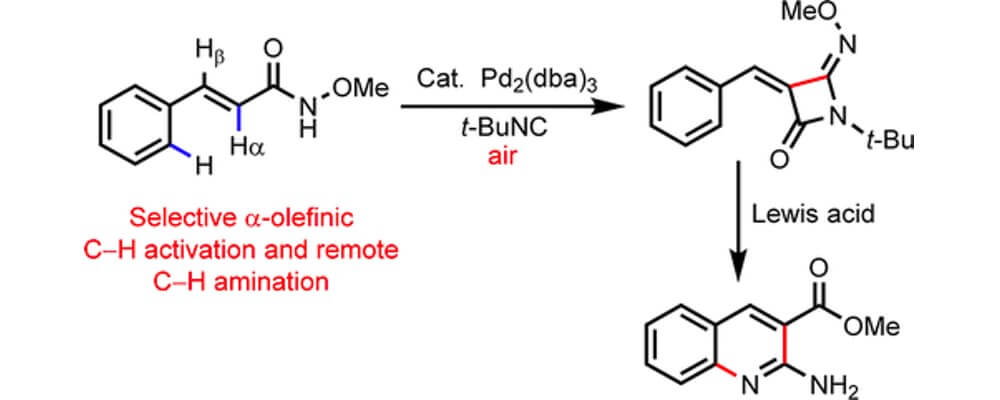
Directed C(sp2)–H functionalization of various aromatic systems has emerged as a powerful means for the construction of aromatic systems, with reliable and efficient means for selective bond forming reactions. This has been extended to beta-olefinic C–H activation via the common five-membered cyclometalation intermediate. In contrast, functionalization at the alpha C–H bond of simple olefins has received only limited attention.
This collaborative report from the Yu and Dai groups builds upon some of their recent work on the development of an electron-rich N-methoxy amide directing group, initially designed to allow C–H activation in the presence of heterocyclic systems, and details an efficient and general activation of the alpha C–H bond of olefinic systems.
This report is of particular import to the pharmaceutical sciences as it is tolerant of a broad scope of heterocyclic substrates and the resulting 4-imino-beta-lactams are not only interesting motifs in their own right, but can be readily transformed to a variety of drug-like structural functionalities.