Ligand-Promoted Borylation of C(sp3)–H Bonds with Palladium(II) Catalysts
J. He, H. Jiang, R. Takise, R.-Y. Zhu, G. Chen, H.-X. Dai, M. Dhar, J. Shi, H. Zhang, P. T. W. Cheng, J.-Q. Yu
Angew. Chem. Int. Ed.,
2016, 55, 2, 785-789; 10.1002/anie.201509996
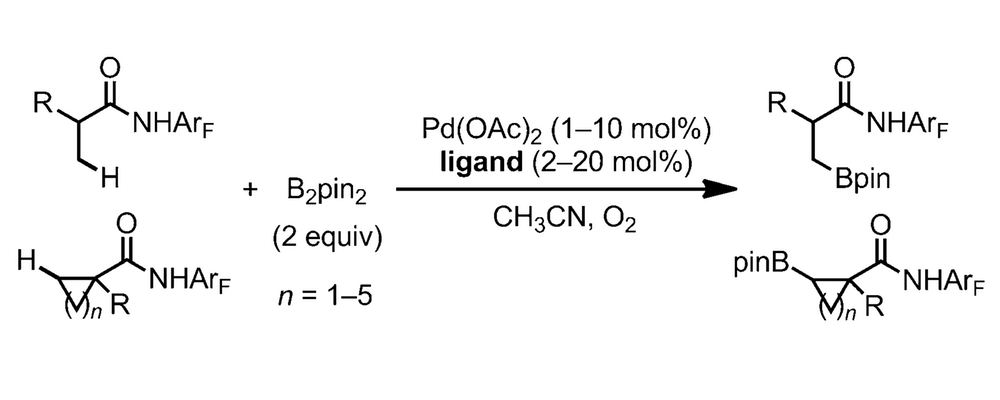
11/2015
The borylation of C–H bonds has become recognized as a flexible and versatile transformation in the field of C–H activation; the newly formed carbon-boron bond can be readily converted into a variety of carbon-carbon or carbon-heteroatom bonds.
This work disclosed by the Yu group, in collaboration with researchers at Bristol-Myers Squib, significantly expands this field through the ligand promoted C–H borylation of C(sp3)-H bonds.
Much of the work on the borylation of C–H bonds has revolved around the use of iridium and rhodium based catalytic systems, with the palladium counterparts proceeding with low conversion. Drawing from their extensive investigation of ligand effects on palladium catalysis the Yu group explored the possibility of optimizing a Pd(II)-catalyzed system, which would proceed through a fundamentally different mechanism and redox-process compared to the established Ir- and Rh-catalyzed transformations.
At the outset of these studies the Yu group identified two significant challenges with this system; the reductive elimination from a Pd-B species is known to be inefficient in the absence of a suitable ligand and the fact that the borylated product has the potential to undergo transmetalation with the Pd(II), leading to de-borylation or beta-hydride elimination side reactions.
This work outlines the studies and optimizations carried out to identify a ligand that establishes a Pd(II)-catalyzed system that is efficient under conditions mild enough to perform the transformation in high yield with low catalyst loadings.
This extension of the C–H borylation chemistry represents a powerful transformation for this field, extending the scope to primary and secondary C(sp3)–H bonds as reaction partners. investigation of stereoselection and further substrate elaboration are underway.