A Diruthenium Catalyst for Selective, Intramolecular Allylic C-H Amination
M. E. Harvey, D. G. Musaev, J. Du Bois
J. Am. Chem. Soc.,
2011, 17207-17216; 10.1021/ja203576p
10/2011
The CCHF brings together experimental and theoretical practitioners to work together and drive forward the understanding and application of C–H Functionalization. This collaborative report from the Du Bois and Muasev groups describes a collaboration that provides a more detailed picture of the role of the catalyst in C–H amination.
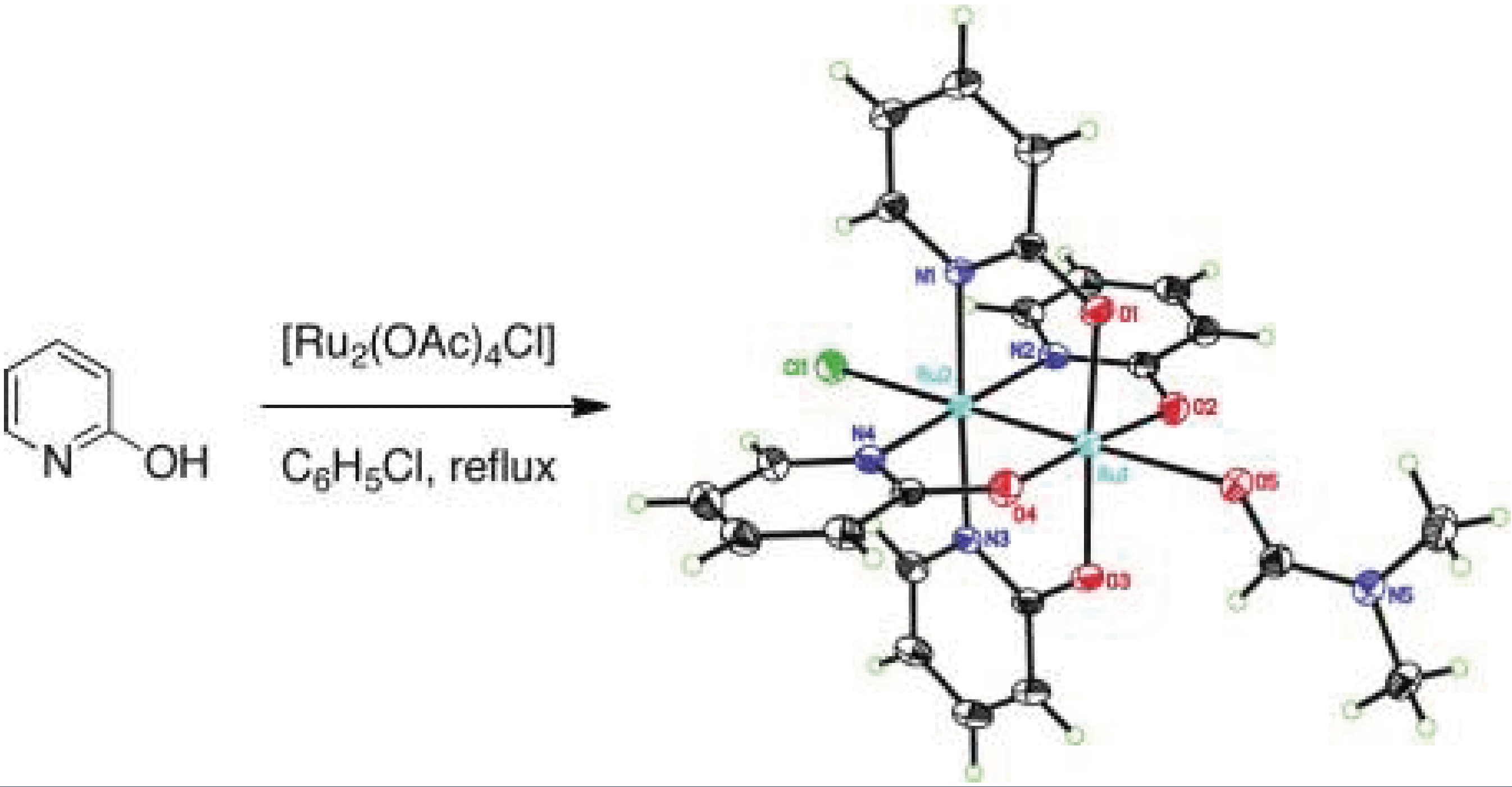
The mixed-valent paddlewheel complex tetrakis(2-oxypyridinato)diruthenium(II,III) chloride, [Ru2(hp)4Cl], catalyzes intramolecular allylic C–H amination with bis(homoallylic) sulfamate esters. These results stand in marked contrast to reactions performed with dirhodium catalysts, which favor aziridine products.
The investigation constitutes the first report of C–H amination using complexes such as [Ru2(hp)4Cl] and related diruthenium adducts. Computational and experimental studies implicate a mechanism for [Ru2(hp)4Cl]-promoted C–H amination involving hydrogen-atom abstraction/radical recombination and the intermediacy of a discrete, albeit short-lived, diradical species. The collective data offer a coherent model for understanding the preference of this catalyst to oxidize allylic (and benzylic) C–H bonds.