Guiding principles for site selective and stereoselective intermolecular C–H functionalization by donor/acceptor rhodium carbenes
Huw M. L. Davies and Daniel Morton
Chem. Soc. Rev,
2011, 40, 1857-1869; 10.1039/C0CS00217H
03/2011
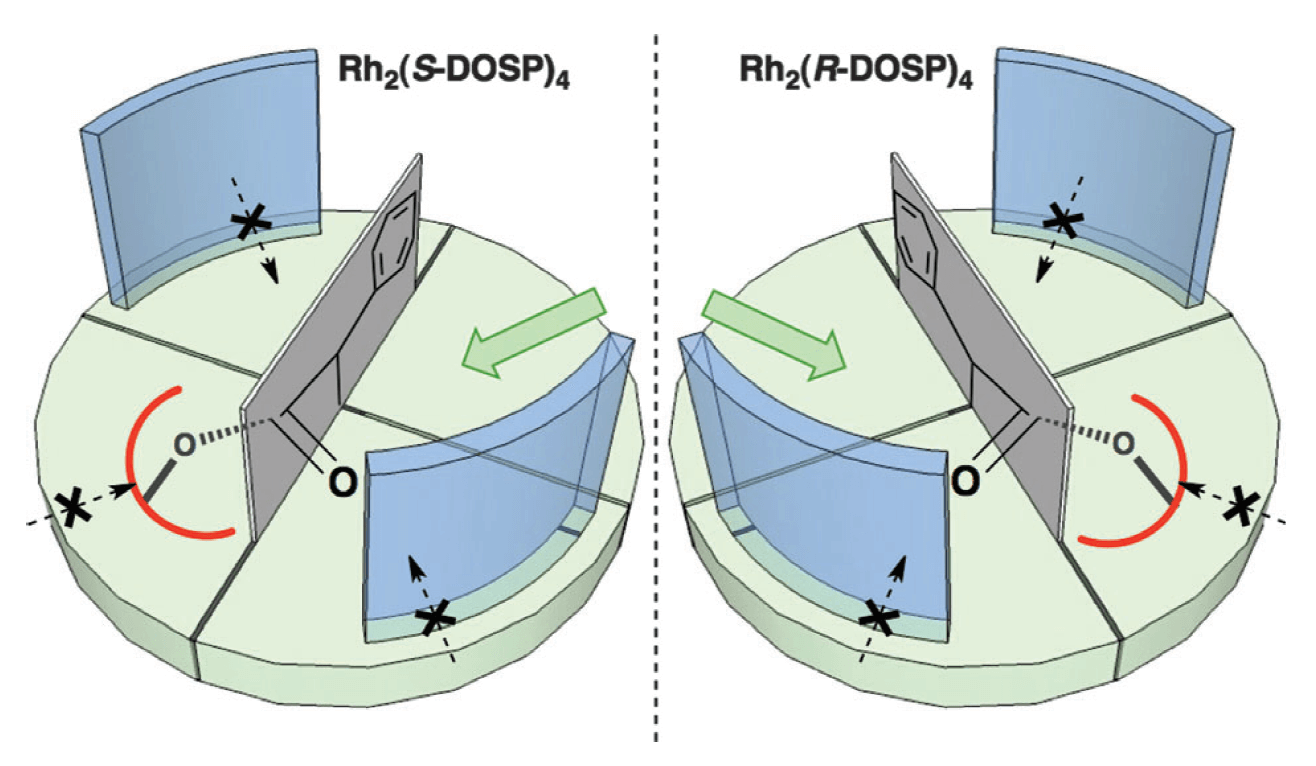
Rhodium(II)-catalyzed carbene C–H insertion has proven to be an excellent method to construct complex molecular skeletons in a rapid and stereocontrolled fashion. The discovery of donor/acceptor substituted carbenes revealed a myriad of opportunities by making the intermolecular reaction possible. This Tutorial review describes the origins and impact of the various factors that dictate the excellent levels of selectivity seen in these reactions.
From the fundamental molecular factors of the substrates to the chiral environment of the paddlewheel ligands, this review provides a detailed explanation of the factors that control both site- and stereoselectivity. It outlines guidelines for future researchers to design and understand their own synthetic strategies based on carbene C–H insertion.